Figure 2. Reaction development.
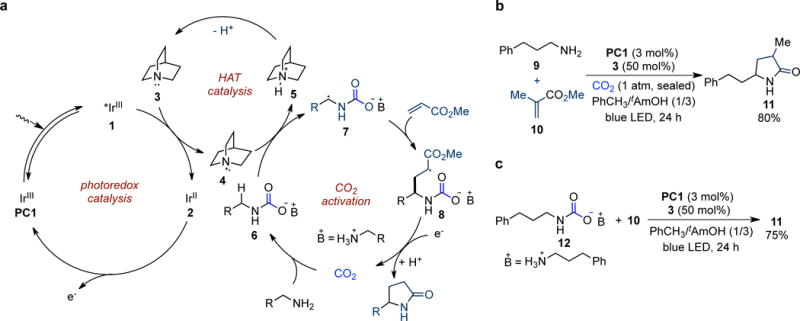
a, Proposed mechanism for the CO2-promoted α–alkylation/lactamization of primary aliphatic amines. One-electron oxidation of quinuclidine 3 by the excited photocatalyst 1 furnishes quinuclidinium radical cation 4, which undergoes selective HAT with the in situ formed alkylammonium carbamate 6 to afford radical 7. Addition into acrylate provides adduct 8, which is reduced by reduced photocatalyst 2 and protonated to give the final γ-lactam product after CO2 dissociation. b, Photocatalyst PC1 and quinuclidine are identified as the optimal catalyst combination in the model reaction using a mixture of toluene and tAmOH as the solvent. c, Reaction using preformed alkylamonium carbamate 12 in the absence of CO2 provides the desired product in 75% yield, which supports our proposal. Isolated yields are shown. tAmOH, tert-amyl alcohol.
