Figure 4. Mechanistic and computational studies.
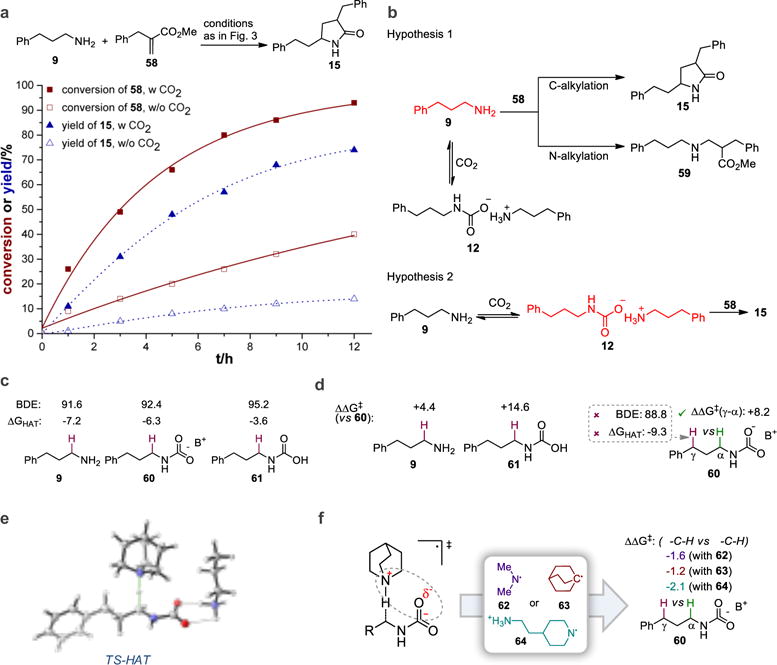
a, 1H nuclear magnetic resonance (NMR) studies show that both acrylate consumption and product formation are faster in the presence of CO2. b, Two hypotheses for the role of CO2 in the reaction. In hypothesis 1, where CO2 is merely a protecting group, acrylate consumption and product formation should be slower in the presence of CO2 due to the lower concentration of free amine 9. In hypothesis 2, where CO2 is an activator, acrylate consumption and product formation should be faster in the presence of CO2 due to the conversion of free amine into more reactive alkylammonium carbamate 12. c, Computed thermodynamic descriptors are unable to explain rate-acceleration by CO2 as well as high α-C−H selectivity. d, Selectivity and reactivity enhancement are achieved in the transition state (TS). e, Computed HAT transition state. f, Computational test for the role of electrostatics - removal or relocation of positive charge in the HAT reagent diminishes site-selectivity. Values are energies in kcal/mol. BDE, bond dissociation energy. B+ = n-C3H7NH3+.
