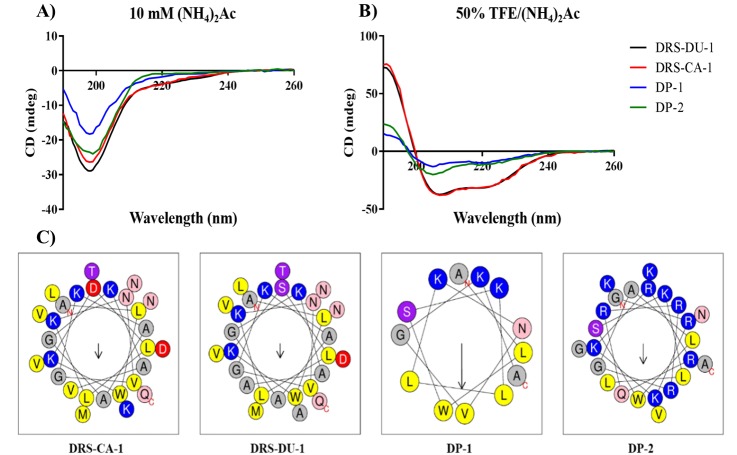
Figure 4. CD spectra of the four peptides (100 µM) in 10 mM ammonium acetate buffer (A) and 50% TFE ammonium acetate buffer (B). Helical wheel projections (Gautier et al., 2008) of peptides (C), with the arrow indicated the direction of the hydrophobic moments.
All the peptides exhibit random coil structure in the aqueous solution while they are able to form α-helical structure in the membrane-mimetic environment. The hydrophobic (yellow), hydrophilic (purple), positively-charged (blue), negatively-charged (red), amide (pink) and small (grey) residues are presented.