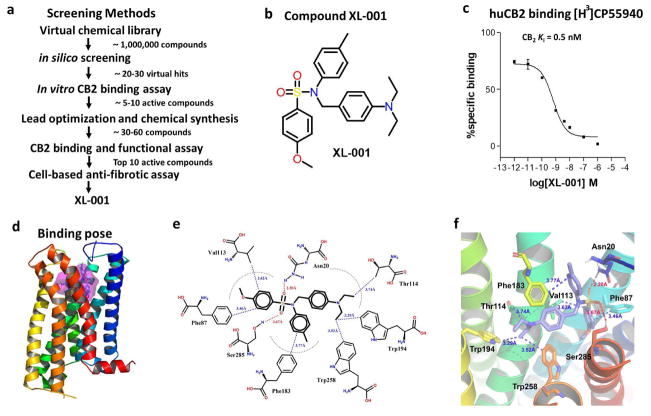
Figure 5.
Discovery and characterization of compound XL-001, a novel inverse agonist of CB2. (a) Screening strategy for identification of XL-001. (b) The chemical structure of XL-001. (c) Binding affinity of XL-001 on the CB2 receptor (CB2 Ki = 0.5 nM). (d) Binding pose of XL-001 with the CB2 receptor. (e, f) The detailed 2D (e) and 3D (f) interactions between XL-001 and CB2. Hydrogen bonding interactions of XL-001 with Asn20 (2.20 Å) and Ser285 (3.67 Å) of CB2. The p-diehtylaminobenzene group formed strong hydrophobic interactions with Thr114 (3.74 Å), Trp194 (3.29 Å), and Trp258 (3.52 Å). Moreover, the p-methoxylbenzene group interacted strongly with Phe87 (3.46 Å) and Val113 (3.62 Å), while the p-methylbenzene group had a strong hydrophobic interaction with Phe183 (3.77 Å).