Abstract
Three new tetra-Schiff bases were synthesized and characterized to be used as photostabilizers for poly(vinyl chloride) (PVC) films. The photostability of PVC films (40 μm thickness) in the presence of Schiff bases (0.5 wt %) upon irradiation (300 h) with a UV light (λmax = 365 nm and light intensity = 6.43 × 10−9 ein∙dm−3∙s−1) was examined using various spectroscopic measurements and surface morphology analysis. The changes in various functional groups’ indices, weight and viscosity average molecular weight of PVC films were monitored against irradiation time. The additives used showed photostability for PVC films, with Schiff base 1 being the most effective additive upon irradiation, followed by 2 and 3. The atomic force microscopy (AFM) images for the PVC surface containing Schiff base 1 after irradiation were found to be smooth, with a roughness factor (Rq) of 36.8, compared to 132.2 for the PVC (blank). Several possible mechanisms that explain PVC photostabilization upon irradiation in the presence of tetra-Schiff bases were proposed.
Keywords: poly(vinyl chloride), Schiff bases, functional group’s indices, atomic force microscopy, photodegradation, irradiation, viscosity average molecular weight
1. Introduction
Polymeric materials are produced in huge quantity and are used in everyday applications. Polyethylene, polypropylene and poly(vinyl chloride) (PVC) are the most produced and used polymers as plastics. The molecular structure of polymeric materials is unique in controlling their chemical, physical and mechanical properties [1,2]. Poly(vinyl chloride) can exit in two common forms, known as rigid and flexible, and has various industrial applications [3]. The rigid PVC can be used in water pipes, windows, doors, synthetic floor tiles, plumbing fittings, phonograph records, and credit cards [4]. The soft PVC can be used as a replacement for rubber in shower curtains, raincoats, and packaging films [4]. Photooxidation can lead to the irreversible deterioration of PVC polymeric materials [5]. Irradiation of PVC with a UV light (250–350 nm) for a long period can lead to a change in color, loss of mechanical and physical properties, reduction in transparency and the formation of cracks and deposits on the surface [6,7,8]. In addition, the PVC photodegradation process can lead to dehydrochlorination and the formation of conjugated double bonds [9]. This is possibly due to impurities during the synthesis process [10], average molecular weight reduction and crosslinking [11,12]. Therefore, the photostability of PVC has to be enhanced for outdoor applications, where the polymeric materials are exposed to light and high temperatures. Various additives at low concentration have been used to enhance the PVC photostability upon UV irradiation [4]. The most common additives include inorganic salts [13,14,15,16], heterocycles [17,18,19,20,21,22,23] and aromatics [24,25,26,27,28]. Schiff bases [29,30,31] and titanium dioxide were found to be the most efficient additives for the photostabilization of PVC [32,33,34].
In this study, we report the successful synthesis of three tetra-Schiff bases, using a simple and efficient procedure, and investigate their PVC photostabilization efficiency as a continuation of our own interest in polymeric materials [35,36,37,38,39,40,41]. The synthesized Schiff bases have been proven to be efficient stabilizers against the photodegradation of PVC samples.
2. Results and Discussion
2.1. Synthesis and Characterization of Schiff Bases 1–3
Schiff bases 1–3 were synthesized based on literature procedures that have been used for the production of 1 [42,43]. Condensation of biphenyl-3,3′,4,4′-tetraamine and excess aromatic aldehydes (four mole equivalents), namely 2-hydroxybenzaldehyde, 3-hydroxybenzaldehyde and 4-nitrobenzaldehyde, in ethanol in the presence of acetic acid as a catalyst under reflux for 4 h, gave the corresponding Schiff bases 1–3 in 78–84% yields (Scheme 1). The physical properties of 1–3 and their elemental analyses are presented in Table 1.
Scheme 1.
Synthesis of Schiff bases 1–3.
Table 1.
Physical properties and elemental analyses for Schiff bases 1–3.
| Schiff Base | Ar | Color | Yield (%) | Mp (°C) | Calcd. (Found; %) | ||
|---|---|---|---|---|---|---|---|
| C | H | N | |||||
| 1 | 2-HOC6H4 | Deep orange | 84 | 234–236 | 8.88 (8.92) | 4.79 (4.85) | 76.17 (76.22) |
| 2 | 3-HOC6H4 | Light brown | 80 | 207–209 | 8.88 (8.91) | 4.79 (4.83) | 76.17 (76.19) |
| 3 | 4-NO2C6H4 | Dark red | 78 | 274–276 | 15.01 (15.23) | 3.51 (3.56) | 64.34 (64.40) |
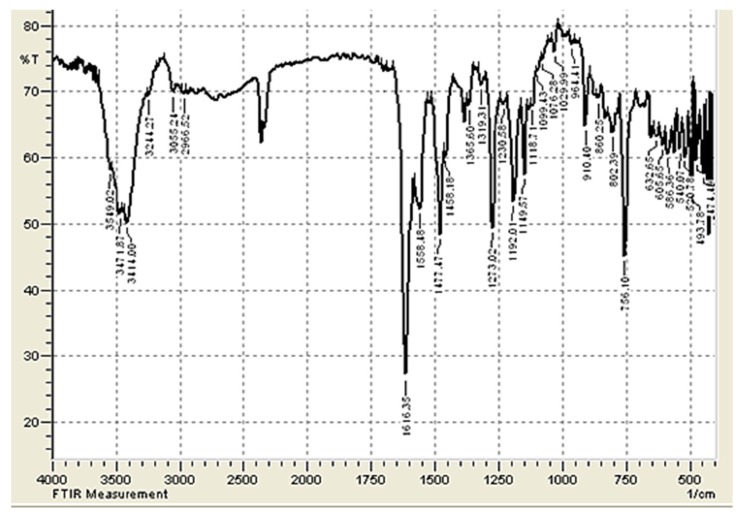
The FT-IR spectra of 1–3 show the presence of intense absorption bands at 1600−1624 cm–1 and can be attributed to the CH=N bonds (Table 2). They also indicate the absence of NH2 group absorption. As an example, Figure 1 shows the FT-IR spectrum for Schiff base 1.
Table 2.
Some FT-IR spectral data for Schiff bases 1–3.
| Schiff Base | FR-IR (υ, cm−1) | |||
|---|---|---|---|---|
| OH | CH=N | C=C (Ar) | C-N | |
| 1 | 3414 | 1616 | 1558 | 1273 |
| 2 | 3417 | 1600 | 1527 | 1273 |
| 3 | - | 1624 | 1516 | 1342 |
Figure 1.
FT-IR spectrum of Schiff base 1.
The 1H-NMR spectra for 1–3 show singlet signals that resonate within the 9.05–8.01 ppm region due to the CH=N protons. They also show all the aromatic protons with the expected multiplicity and chemical shifts (Table 3).
Table 3.
1H-NMR spectral data for Schiff bases 1–3.
| Schiff Base | 1H-NMR (400 MHz: DMSO-d6, δ, ppm, J in Hz) |
|---|---|
| 1 | 9.17 (s, exch., 4 H, OH), 9.08 (s, 4 H, CH), 7.93 (s, 2 H, Ar), 7.71 (d, J = 8.2 Hz, 4 H, Ar), 7.64 (d, J = 8.5 Hz, 2 H, Ar), 7.44 (t, J = 8.2 Hz, 4 H, Ar), 7.25 (d, J = 8.5 Hz, 2 H, Ar), 7.00 (t, J = 8.2 Hz, 4 H, Ar), 6.99 (d, J = 8.2 Hz, 4 H, Ar) |
| 2 | 8.08 (s, exch., 4 H, OH), 8.01 (s, 4 H, CH), 7.63 (s, 2 H, Ar), 7.53 (d, J = 8.1 Hz, 4 H, Ar), 7.48 (d, J = 8.5 Hz, 2 H, Ar), 7.48 (s, 4 H, Ar), 7.35 (d, J = 8.5 Hz, 2 H, Ar), 7.18 (t, J = 8.1 Hz, 4 H, Ar), 6.95 (d, J = 8.1 Hz, 4 H, Ar) |
| 3 | 8.61 (s, 4 H, CH), 8.32 (d, J = 8.3 Hz, 8 H, Ar), 8.05 (d, J = 8.3 Hz, 8 H, Ar), 7.70 (s, 2 H, Ar), 7.50 (d, J = 8.4 Hz, 2 H, Ar), 7.35 (d, J = 8.4 Hz, 2 H, Ar) |
2.2. Photodegradation of PVC Films by FT-IR Spectroscopy
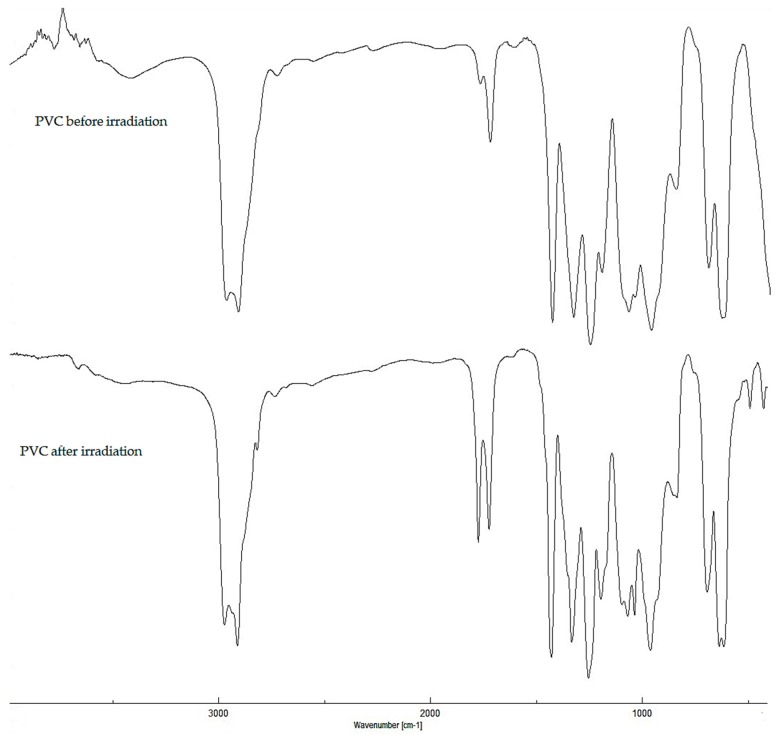
Previous reports show that the most effective concentration of Schiff bases to be added to stabilize PVC was 0.5 wt % [21]. Therefore, Schiff bases 1–3 (0.5 wt %) were used as additives to improve the photostability of PVC polymeric materials. The PVC films (40 μm thickness) were irradiated with UV light (λmax = 365 nm and light intensity = 6.43 × 10−9 ein∙dm−3∙s−1) for up to 300 h. The FT-IR spectroscopy (400–4000 cm−1) was used to evaluate the changes in certain functional groups within the PVC films. The photostability of PVC films was studied by monitoring the changes in intensity for polyene (1602 cm−1), carbonyl (1722 cm−1), and hydroxyl (3500 cm−1) peaks, as compared to a standard peak (1328 cm−1) upon irradiation [44]. The FT-IR spectra of pure PVC films before and after irradiation (300 h) are shown in Figure 2.
Figure 2.
FT-IR spectrum of poly(vinyl chloride) (PVC) films before and after irradiation.
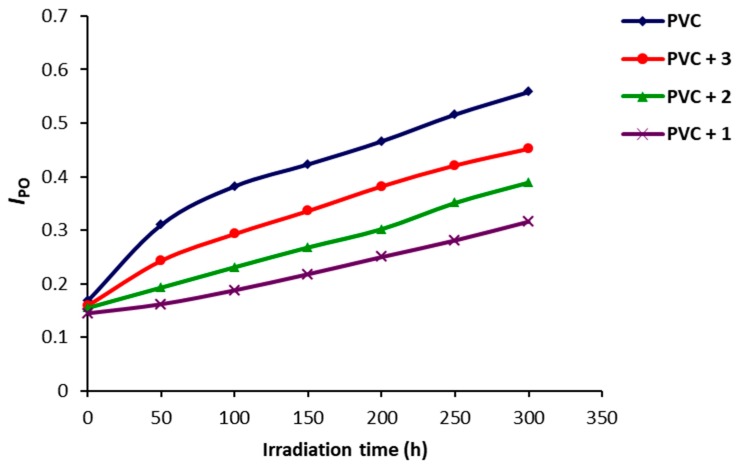
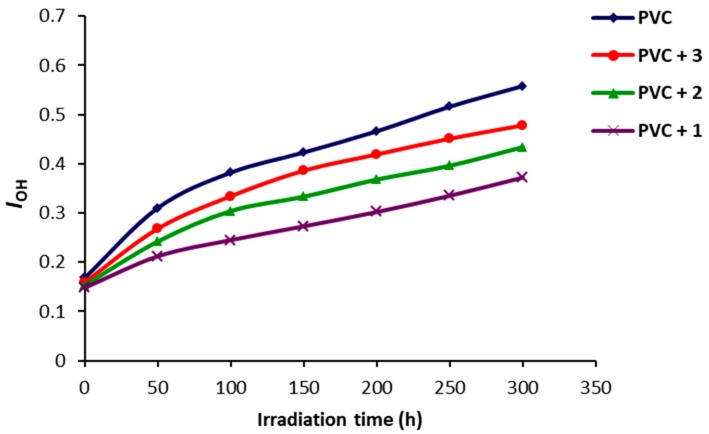
The carbonyl (ICO), polyene (IPO) and hydroxyl (IOH) indices were calculated and plotted against irradiation time (Figure 3, Figure 4 and Figure 5). Such indices were lower for the PVC films containing Schiff bases 1–3 compared to the ones for the blank PVC. Clearly, Schiff bases 1–3 and in particular 1 could be used as efficient photostabilizers for PVC films.
Figure 3.
Effect of irradiation of PVC films on ICO.
Figure 4.
Effect of irradiation of PVC films on IPO.
Figure 5.
Effect of irradiation of PVC films on IOH.
2.3. Photodegradation of PVC Films by Weight Loss
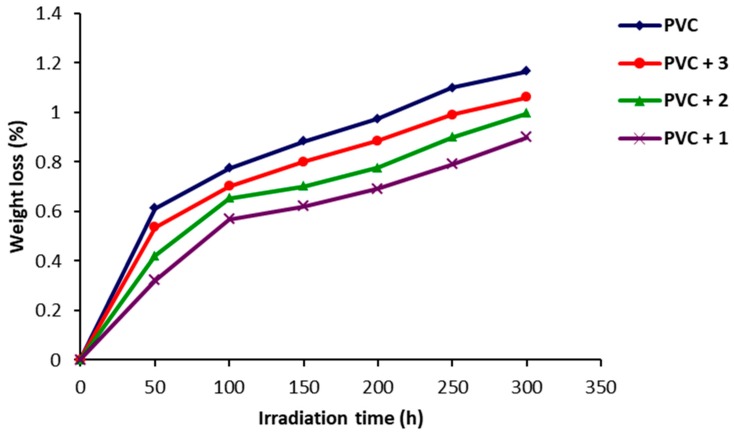
Dehydrochlorination, elimination of hydrochloride (HCl), is a major cause of PVC photodegradation and leads to weight loss [45]. The degree of photodegradation of PVC can be measured from the percentage of PVC weight loss after irradiation. The effect of irradiation time on the percentage of PVC films weight loss is shown in Figure 6. Clearly, the weight loss in PVC was lower in the presence of Schiff bases 1–3 compared to the blank PVC film. Schiff base 1 was the most efficient additive since it could act as an effective radical scavenger, possibly due to the presence of the ortho-hydroxyl group, which increases the stability through resonance. During irradiation of PVC films containing Schiff bases, not only was weight loss reduced but the discoloration was less noticeable [46].
Figure 6.
Effect of irradiation of PVC films on weight loss (%).
2.4. Photodegradation of PVC Films by Variation in Molecular Weight
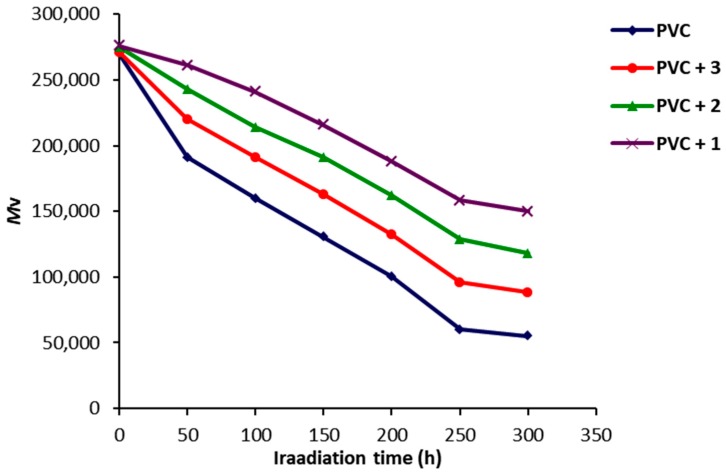
The Mark–Houwink equation [44,47,48] was used to calculate the viscosity average molecular weight . Viscosity average molecular weight is directly proportional to the intrinsic viscosity [η] [49]. The variation in for PVC films containing 1–3 (0.5 wt %) during the irradiation process was measured (Figure 7). The decrease in was higher for the blank PVC film compared to the ones containing 1–3, in which Schiff base 1 was again the most efficient additive. The reduction in during the irradiation process may be due to the degradation of PVC chains producing low molecular weight polymeric chains [50,51].
Figure 7.
Effect of irradiation of PVC films on .
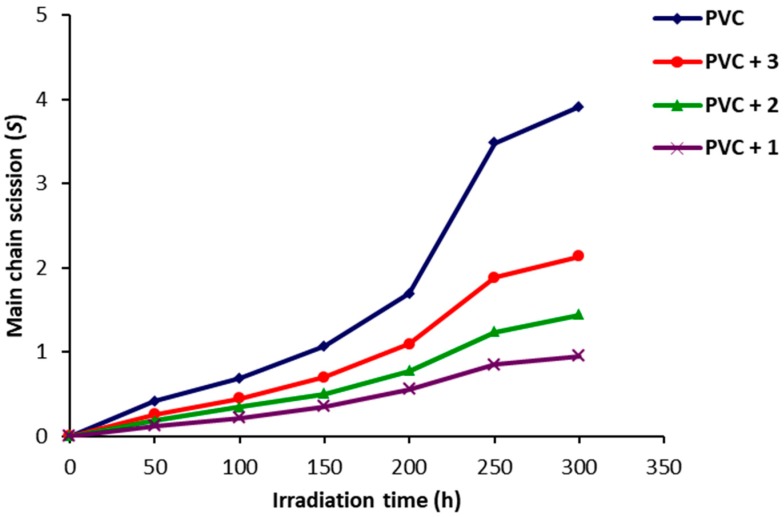
During the photolysis process, insoluble residue in tetrahydrofuran (THF) was observed and was believed to be due to the PVC chains branching or crosslinking [52]. To confirm this conclusion, the average chain scission (S) was calculated using Equation (1) and plotted against irradiation time (Figure 8). The S value is dependent on the viscosity average molecular weight at irradiation time 0 ( and t (). The results indicate that the degree of branching and/or crosslinking was higher for the PVC (blank) compared to the ones containing Schiff bases. The S values for the PVC film containing Schiff base 1 were minimal.
| (1) |
Figure 8.
Effect of irradiation of PVC films on S.
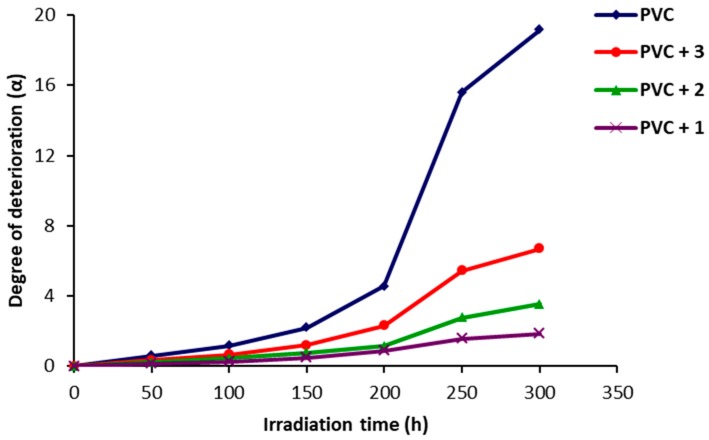
At the initial stage of PVC photodegradation, weak bonds, randomly distributed along the polymeric chains, break dawn rapidly. The degree of PVC photodegradation is directly proportional to the degree of deterioration (α). The α values were calculated using Equation (2) from , S and molecular weight (m). The increase in α for the PVC samples was very low in the first 150 h and became sharp afterwards (Figure 9). The α values for the PVC samples containing 1–3 were very low compared to the ones obtained for the blank PVC sample.
| (2) |
Figure 9.
Effect of irradiation of PVC films on α.
2.5. Surface Morphology of PVC Films
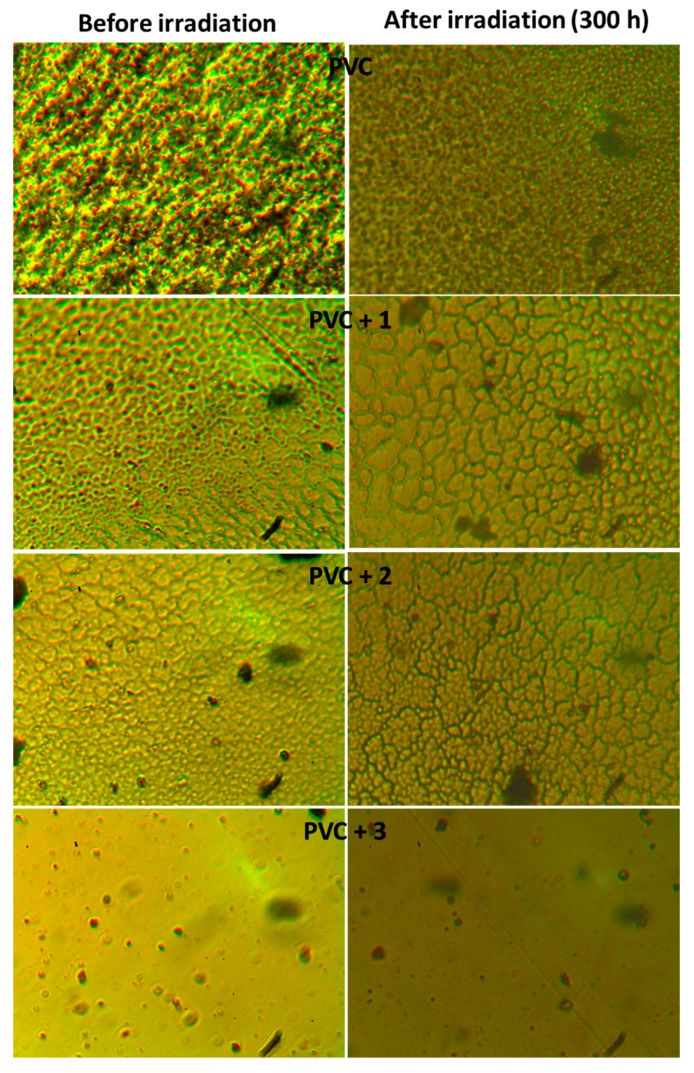
The surface morphology provides information about defects, roughness, and irregularity within the polymeric materials [53]. Also, it can detect the changes in polymer surface upon irradiation where decomposition takes place as chain scission [54]. The surface morphology images for PVC films before and after irradiation (300 h) are shown in Figure 10. Compared to before irradiation, the PVC (blank) image shows the presence of grooves, white spots and cracks on the surface following irradiation. Also, the surface was rough and underwent a color change. Such surface irregularity is a result of photodegradation of PVC due to dehydrochlorination. Comparatively, the images for PVC films containing Schiff bases, in particular 1, were nearly smooth and have only a few white spots. Clearly, 1–3 can inhibit elimination of HCl and enhance the photostability of PVC films upon irradiation.
Figure 10.
Effect of irradiation of PVC films on surface morphology.
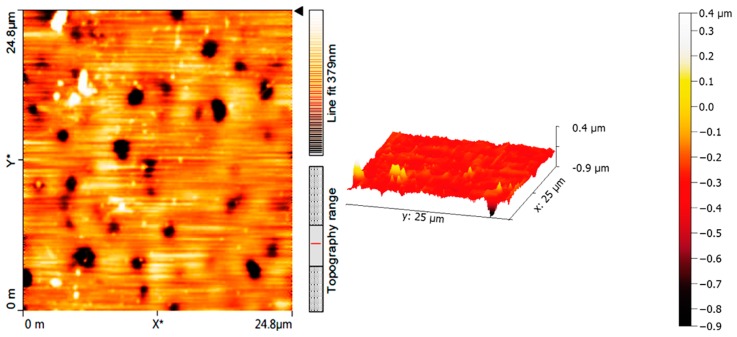
Atomic force microscopy (AFM) provides two- and three-dimensional images for sample surface and does not require a vacuum environment. It is a simple technique that can be applied in various fields [55]. The 2D and 3D AFM images for the PVC film (bank) which contain Schiff base 1 (0.5 wt %) after irradiation (300 h) are shown in Figure 11 and Figure 12, respectively. The AFM images for the surface of the PVC film (area = 5.0 × 5.0 μm2) containing Schiff base 1 after irradiation show it to be very smooth with a low roughness factor (Rq = 36.8 nm). Comparatively, the surface of the PVC (blank) film was very rough (Rq = 132.2). The rough surface of the blank PVC film after irradiation could be due to bonds breaking [56] and/or dehydrochlorination [57]. Dehydrochlorination of PVC usually takes place at high temperatures [57].
Figure 11.
2D and 3D atomic force microscopy (AFM) images of PVC film (blank) after irradiation (300 h).
Figure 12.
2D and 3D AFM images of PVC film containing 1 after irradiation (300 h).
2.6. Proposed Mechanisms of PVC Film Photostabilization
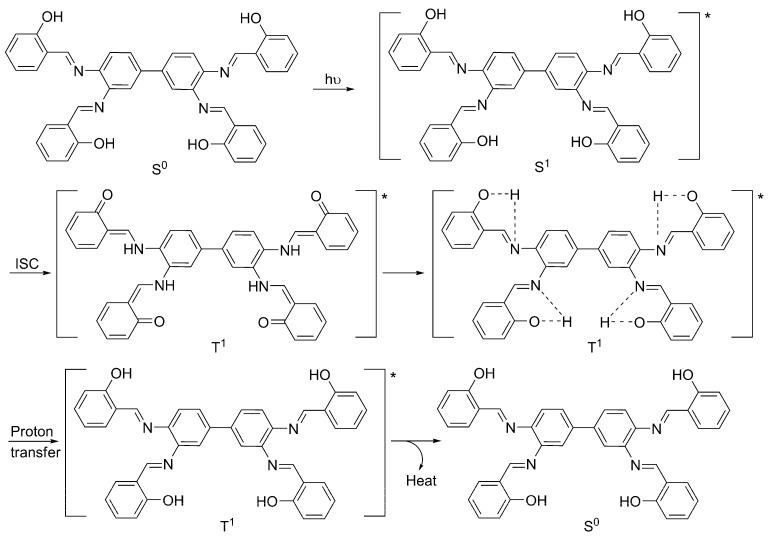
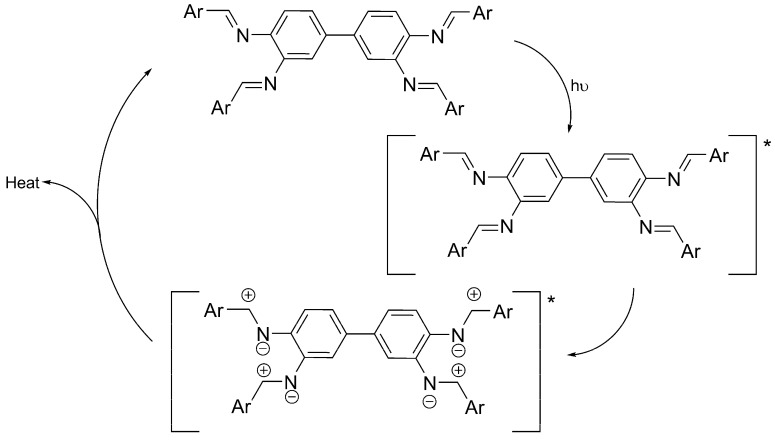
The synthesized Schiff bases 1–3 act as photostabilizers for PVC films upon irradiation with a UV light. The results show that the efficiency of such additives follows the order 1 > 2 > 3. Various mechanisms could be suggested to explain the role that Schiff bases 1–3 play in the photostabilization of PVC films. The performance of compounds 1–3 varies in their stabilization effect on PVC films due to the nature and position of substituents on the aryl rings. Schiff base 1 was the most efficient additive to stabilize PVC films. This is possibly due to the hydroxyl groups at the ortho-positions of the aryl rings. Schiff bases can dissipate the UV radiation into harmless heat via a proton transfer for the Schiff base singlet excited state (S1 *) and internal conversion (IC), followed by proton transfer. Also, it is possible that the UV energy could be released as harmless heat via intersystem conversion (ISC) of the additive S1 * state to the triplet excited state (T1 *), followed by a proton transfer, followed by conversion to the ground state (S0) [19,30]. Scheme 2 represents the possibility of PVC photostabilization via a proton transfer and ISC in the presence of Schiff base. For most photochemical reactions, only the lowest S1 * and T1 * states have to be considered for the initiation step [58]. Excited singlet states are short-lived species with a lifetime in the range 10−9–10−6 s. Photodegradation of polymers often proceeds from T1 * states which have a longer lifetime than S1 * states, since the return to a S0 state is spin-forbidden [58]. The bond breaking within polymeric chains only takes place when the bond dissociation energy is lower than the excitation energy, or when the excited state is a repulsive one.
Scheme 2.
PVC photostabilization via a proton transfer and intersystem conversion in the presence of 1. * represents the excited state.
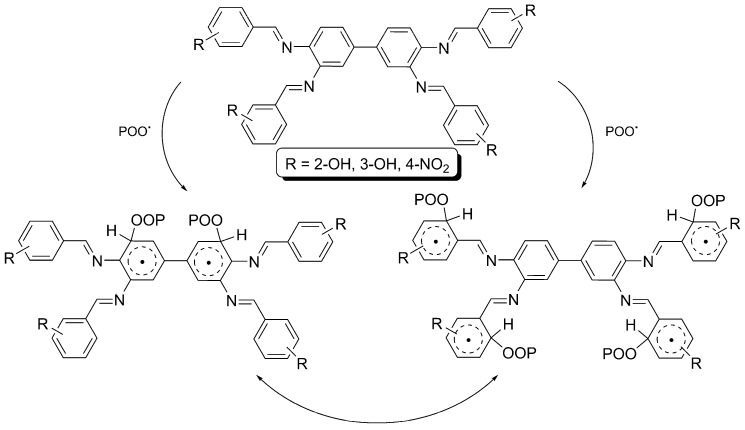
Schiff bases 1–3 could act as radical scavengers in the presence of a chromophore (POO•) to stabilize the PVC. When excited, a stable complex between the chromophore and Schiff base could be formed, in which energy can be transferred. The stable charge transfer complex would be stabilized via resonance of aryl rings within the Schiff bases (Scheme 3) [59].
Scheme 3.
PVC photostabilization via a radical scavenger in the presence of 1–3.
Tetra-Schiff bases 1–3 are electron rich (six aryl rings) and can absorb the UV radiation energy directly and dissipate it into harmless heat energy (Scheme 4). Aromatic compounds commonly act as UV absorbers [60].
Scheme 4.
PVC photostabilization of PVC via direct absorption of UV radiation. * represents the excited state.
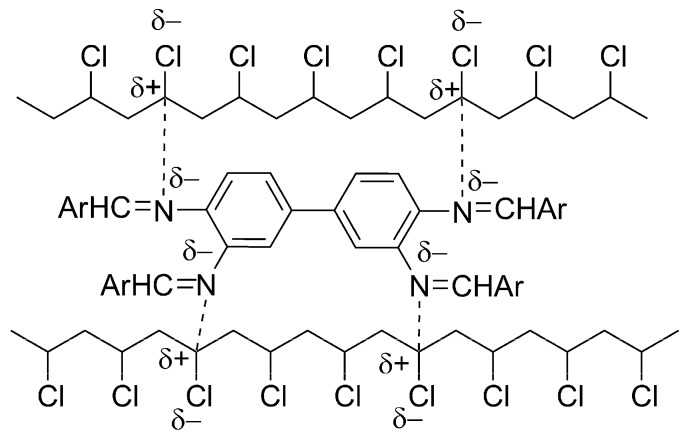
The possible attraction between polarized CH=N bonds within Schiff bases 1–3 and polarized C-Cl bonds within PVC chains could stabilize PVC (Scheme 5). Such attraction could allow efficient transfer of the PVC excited energy to Schiff bases and then to a harmless level of energy.
Scheme 5.
PVC photostabilization via interaction between PVC films and Schiff bases.
3. Experimental Section
3.1. General
Chemicals were obtained from Sigma-Aldrich Chemical Company (Gillingham, UK). Melting points were recorded on a hot stage Gallenkamp melting point apparatus. The Fourier transform Infrared (FT-IR) spectra (400–4000 cm−1) were recorded on a Shimadzu 8400 Spectrophotometer (Shimadzu Cooperation, Kyoto, Japan) using the KBr disk technique. Proton nuclear magnetic resonance (1H-NMR) spectra (400 MHz) were recorded on a Bruker DRX400 NMR Spectrometer (Bruker, Zürich, Switzerland) in DMSO-d6 related to tetramethylsilane. The elemental analyses were performed on the Vario EL III Elementar instrument. An accelerated weather-meter QUV tester (Q-Panel Company, Homestead, FL, USA) was used to irradiate PVC films at room temperature with a UV light (λmax = 365 nm and light intensity = 6.43 × 10−9 ein∙dm−3∙s−1). The morphology images of PVC films were recorded on Meiji Techno Microscope (Meiji Techno, Tokyo, Japan). Atomic force microscopy (AFM) images were recorded on the Veeco instrument (Veeco Instruments Inc., Plainview, New York, NY, USA).
3.2. Synthesis of Schiff Bases 1–3
A mixture of biphenyl-3,3′,4,4′-tetraamine (2.14 g, 10.0 mmol) and appropriate aromatic aldehyde (40.0 mmol) in anhydrous ethanol (25 mL) in the presence of glacial acetic acid (0.5 mL) was stirred magnetically under reflux for 4 h. The mixture was left to cool down to room temperature and the solid obtained was filtered, washed with ethanol and dried to give pure 1–3 in 78–84% yields.
3.3. PVC Films Preparation
The PVC films were prepared using a solution-cast technique at room temperature. Schiff bases 1–3 were added to PVC at a concentration of 0.5% by weight in THF. The solvent was removed by evaporation at room temperature for 24 h. The thickness of PVC films (40 µm) was fixed using a Digital Vernier Caliper 2610A micrometer (Vogel GmbH, Kevelaer, Germany).
3.4. Light Exposure
A UV Light (λmax = 365 nm and light intensity = 6.2 × 10−9 ein∙dm−3∙s−1 nm) was used to irradiate the PVC using an accelerated weather-meter QUV tester (Philips, Saarbrücken, Germany) at room temperature. The PVC films were rotated occasionally to ensure that the incident light intensity was the same on all sides.
3.5. Photodegradation of PVC Films by FT-IR Spectrophotometry
The changes in IR absorption bands for carbonyl (1722 cm−1), polyene (1602 cm−1) and hydroxyl (3500 cm−1) groups were monitored and compared to a reference peak (1328 cm−1). The indices for carbonyl (IC=O), polyene (IC=C) and hydroxyl (IOH) groups were calculated using Equation (3). The functional group index (Is) is dependent on both the absorbance peak during the study (As) and that of the reference peak (Ar) [44].
| (3) |
3.6. Photodegradation of PVC Films by Weight Loss
The weight loss percentage of PVC samples during the irradiation process was calculated by the weight of the PVC sample before (W1) and after irradiation (W2) using Equation (4).
| (4) |
3.7. Photodegradation of PVC Films by Viscometry Method
The Mark–Houwink relation, Equation (5), was used to calculate the PVC films’ average molecular weight () in solution (g/100 mL) using an Ostwald U-tube viscometer [50]. is highly dependent on the intrinsic viscosity [η] of PVC and constants (α and K) related to the polymer–solvent system.
| (5) |
The relative viscosity (ηre) at a flow time t0 (pure solvent) and specific viscosity (ηsp) were calculated using Equations (6) and (7) in which the flow time for pure solvent (THF) and PVC sample was t0 and t, respectively.
| (6) |
| (7) |
The intrinsic viscosity was calculated using Equation (8) in which C is the PVC concentration in THF (g/100 mL).
| (8) |
Molecular weight of PVC samples was calculated from [η] using Equation (9).
| (9) |
4. Conclusions
New tetra-Schiff bases which are highly aromatic were synthesized and characterized using various techniques. The photostability of PVC films was increased in the presence of new Schiff bases. The Schiff bases act as photostabilizers for PVC through direct absorption of the UV radiation, proton transfer and intersystem crossing, radical scavengers or an interaction with PVC chains. The photostabilization activity of PVC films containing additives follows the order 1 > 2 > 3.
Acknowledgments
The project was supported by King Saud University, Deanship of Scientific Research, Research Chairs and Al-Nahrain and Tikrit Universities.
Author Contributions
E.Y., A.S.H., A.A. and G.A.E.-H. conceived and designed the experiments. D.S.A. performed the experiments and analyzed the data. G.A.E.-H., E.Y., A.S.H. and A.A. wrote the paper. All authors discussed the results and improved the final text of the paper.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the tetra-Schiff bases are available from the authors.
References
- 1.National Research Council . Polymer Science and Engineering: The Shifting Research Frontiers. The National Academies Press; Washington, DC, USA: 1994. Manufacturing: Materials and processing; pp. 65–115. Chapter 3. [Google Scholar]
- 2.Braun D. Thermal degradation of poly (vinyl chloride) In: Grassie N., editor. Developments in Polymer Degradation. Applied Science Publishers; London, UK: 1981. [Google Scholar]
- 3.Allsopp M.W., Vianello G. Ullmann’s Encyclopedia of Industrial Chemistry. Wiley-VCH Verlag GmbH & Co. KGaA; Weinheim, Germany: 2012. Poly(vinyl chloride) [Google Scholar]
- 4.Titow W.V. PVC Technology. 4th ed. Elsevier Applied Science Publishers; London, UK: 1984. [Google Scholar]
- 5.Grassie N., Scott G. Degradation and Stabilization of Polymers. Cambridge University Press; London, UK: 1985. [Google Scholar]
- 6.Nicholson J.W. The Chemistry of Polymers. 3rd ed. RSC Publisher; Cambridge, UK: 2012. [Google Scholar]
- 7.Decker C. In: Degradation and Stabilisation of PVC. Owen E.D., editor. Springer; Dordrecht, The Netherlands: 1984. [Google Scholar]
- 8.Gewert B., Plassmann M.M., MacLeod M. Pathways for degradation of plastic polymers floating in the marine environment. Environ. Sci. Proc. Impacts. 2015;17:1513–1521. doi: 10.1039/C5EM00207A. [DOI] [PubMed] [Google Scholar]
- 9.Iván B., Kennedy J.P., Kélen T., Tüdòs F., Nagy T.T., Turcsanyi B. Degradation of PVCs obtained by controlled chemical dehydrochlorination. J. Polym. Sci. Pol. Chem. 1983;21:2177–2188. doi: 10.1002/pol.1983.170210802. [DOI] [Google Scholar]
- 10.Caraculacu A., Bezdadea E.C., Istrate G. Structure of branching in PVC. J. Polym. Sci. Pol. Chem. 1970;8:1239–1246. doi: 10.1002/pol.1970.150080516. [DOI] [Google Scholar]
- 11.Starnes W.H. Structural and mechanistic aspects of the thermal degradation of poly(vinyl chloride) Prog. Polym. Sci. 2002;27:2133–2170. doi: 10.1016/S0079-6700(02)00063-1. [DOI] [Google Scholar]
- 12.Fahmy M.M., Mohamed R.R., Mohamed N.A. Novel antimicrobial organic thermal stabilizer and co-stabilizer for rigid PVC. Molecules. 2012;17:7927–7940. doi: 10.3390/molecules17077927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deanin R.D., Reynolds H.H., Ozcayir Y. Thermal stabilization of polyvinyl chloride by group II metal laurates. J. Appl. Polym. Sci. 1969;13:1247–1252. doi: 10.1002/app.1969.070130612. [DOI] [Google Scholar]
- 14.Birmingham J.N. The effect of surface oxidation and titanium dioxide on exterior PVC color retention. J. Vinyl Addit. Technol. 1995;1:84–87. doi: 10.1002/vnl.730010208.n. [DOI] [Google Scholar]
- 15.Cheng Q., Li C., Pavlinek V., Saha P., Wang H. Surface-modified antibacterial TiO2/Ag+ nanoparticles: Preparation and properties. Appl. Surf. Sci. 2006;252:4154–4160. doi: 10.1016/j.apsusc.2005.06.022. [DOI] [Google Scholar]
- 16.Folarin O.M., Sadiku E.R. Thermal stabilizers for poly(vinyl chloride): A review. Int. J. Phys. Sci. 2011;6:4323–4330. [Google Scholar]
- 17.Bojinov V.B., Grabchev I.K. Novel functionalized 2-(2-hydroxyphenyl)-benzotriazole-benzo[de]isoquinoline-1,3-dione fluorescent UV absorbers: Synthesis and photostabilizing efficiency. J. Photochem. Photobiol. A. 2005;172:308–315. doi: 10.1016/j.jphotochem.2004.12.020. [DOI] [Google Scholar]
- 18.Sabaa M.W., Oraby E.H., Naby A.S.A., Mohamed R.R. N-Phenyl-3-substituted-5-pyrazolone derivatives as organic stabilizer for rigid PVC against photodegradation. J. Appl. Polym. Sci. 2005;101:1543–1555. doi: 10.1002/app.23402. [DOI] [Google Scholar]
- 19.Yousif E., Salih N., Salimon J. Improvement of the photostabilization of PVC films in the presence of 2N-salicylidene-5-(substituted)-1,3,4-thiadiazole. J. Appl. Polym. Sci. 2011;120:2207–2214. doi: 10.1002/app.33463. [DOI] [Google Scholar]
- 20.Yousif E., Hameed A., Salih N., Salimon J., Abdullah B. New photostabilizers for polystyrene based on 2,3-dihydro-(5-mercapto-1,3,4-oxadiazol-2-yl)-phenyl-2-(substituted)-1,3,4-oxazepine-4,7-dione compounds. SpringerPlus. 2013;2:104. doi: 10.1186/2193-1801-2-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balakit A.A., Ahmed A., El-Hiti G.A., Smith K., Yousif E. Synthesis of new thiophene derivatives and their use as photostabilizers for rigid poly(vinyl chloride) Int. J. Polym. Sci. 2015;2015:510390. doi: 10.1155/2015/510390. [DOI] [Google Scholar]
- 22.Ali M.A., El-Hiti G.A., Yousif E. Photostabilizing efficiency of poly(vinyl chloride) in the presence of organotin(IV) complexes as photostabilizers. Molecules. 2016;21:1151. doi: 10.3390/molecules21091151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yousif E., Hameed A., Rasheed R., Mansoor H., Farina Y., Graisa A., Salih N., Salimon J. Synthesis and photostability study of some modified poly(vinyl chloride) containing pendant benzothiazole and benzimidozole ring. Int. J. Chem. 2010;2:65–80. doi: 10.5539/ijc.v2n1p65. [DOI] [Google Scholar]
- 24.Sabaa M.W., Mikhael M.G., Mohamed N.A., Yassin A.A. N-Substituted maleimides as thermal stabilizers for rigid poly (vinyl chloride) Angew. Makromol. Chem. 1989;168:23–35. doi: 10.1002/apmc.1989.051680103. [DOI] [Google Scholar]
- 25.Sabaa M.W., Oraby E.H., Abdel Naby A.S., Mohammed R.R. Anthraquinone derivatives as organic stabilizers for rigid poly(vinyl chloride) against photo-degradation. Eur. Polym. J. 2005;41:2530–2543. doi: 10.1016/j.eurpolymj.2005.05.015. [DOI] [Google Scholar]
- 26.Zhao Y., Dan Y. Preparation and characterization of a high molecular weight UV-stabilizer based on a derivative of 2,4-dihydroxybenzophenone and its application in polymer materials. J. Appl. Polym. Sci. 2006;102:2203–2211. doi: 10.1002/app.24286. [DOI] [Google Scholar]
- 27.Tomohito K., Masahiko O., Guido G., Tadaaki M., Toshiaki Y. Antibacterial effect of thiocyanate substituted poly (vinyl chloride) J. Polym. Res. 2011;18:945–947. [Google Scholar]
- 28.Tomi I.H.R., Ali G.Q., Jawad A.H., Yousef E. Synthesis and characterization of gallic acid derivatives and their utilized as organic photo-stabilizers for poly (vinyl chloride) J. Polym. Res. 2017;24:119. doi: 10.1007/s10965-017-1283-7. [DOI] [Google Scholar]
- 29.Yousif E., Hasan A., El-Hiti G.A. Spectroscopic, physical and topography of photochemical process of PVC films in the presence of Schiff base metal complexes. Polymers. 2016;8:204. doi: 10.3390/polym8060204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yousif E., El-Hiti G.A., Hussain Z., Altaie A. Viscoelastic, spectroscopic and microscopic study of the photo irradiation effect on the stability of PVC in the presence of sulfamethoxazole Schiff’s bases. Polymers. 2015;7:2190–2204. doi: 10.3390/polym7111508. [DOI] [Google Scholar]
- 31.Yousif E., Al-Amiery A.A., Kadihum A., Kadhum A.H., Mohamad A. Photostabilizing efficiency of PVC in the presence of Schiff bases as photostabilizers. Molecules. 2015;20:19886–19899. doi: 10.3390/molecules201119665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hussain Z., El-Hiti G.A., Ahmed A., Altaee N., Yousif E. Photocatalytic degradation of polyhydroxybutyrate films using titanium dioxide nanoparticles as a photocatalyst. Russ. J. Appl. Chem. 2016;89:1536–1543. doi: 10.1134/S1070427216090238. [DOI] [Google Scholar]
- 33.Kockler J., Oelgemöller M., Robertson S., Glass B.D. Influence of titanium dioxide particle size on the photostability of the chemical UV-filters butyl methoxy dibenzoylmethane and octocrylene in a microemulsion. Cosmetics. 2014;1:128–139. doi: 10.3390/cosmetics1020128. [DOI] [Google Scholar]
- 34.Farrar R.R., Jr., Shapiro M., Javaid I. Photostabilized titanium dioxide and a fluorescent brightener as adjuvants for a nucleopolyhedrovirus. BioControl. 2003;48:543–560. doi: 10.1023/A:1025723316426. [DOI] [Google Scholar]
- 35.Smith K., Balakit A.A., Pardasani R.T., El-Hiti G.A. New polymeric sulfide-borane complexes: Convenient hydroborating and reducing reagents. J. Sulfur Chem. 2011;32:287–295. doi: 10.1080/17415993.2011.590489. [DOI] [Google Scholar]
- 36.Smith K., Balakit A.A., El-Hiti G.A. Poly(propylene sulfide)-borane: Convenient and versatile reagent for organic synthesis. Tetrahedron. 2012;68:7834–7839. doi: 10.1016/j.tet.2012.07.037. [DOI] [Google Scholar]
- 37.Yousif E., El-Hiti G.A., Haddad R., Balakit A.A. Photochemical stability and photostabilizing efficiency of poly(methyl methacrylate) based on 2-(6-methoxynaphthalen-2-yl) propanoate metal ion complexes. Polymers. 2015;7:1005–1019. doi: 10.3390/polym7061005. [DOI] [Google Scholar]
- 38.Smith K., Al-Zuhairi A.J., El-Hiti G.A., Alshammari M.B. Comparison of cyclic and polymeric disulfides as catalysts for the regioselective chlorination of phenols. J. Sulfur Chem. 2015;36:74–85. doi: 10.1080/17415993.2014.965170. [DOI] [Google Scholar]
- 39.Altaee N., El-Hiti G.A., Fahdil A., Sudesh K., Yousif E. Biodegradation of different formulations of polyhydroxybutyrate films in soil. SpringerPlus. 2016;5:762. doi: 10.1186/s40064-016-2480-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Altaee N., El-Hiti G.A., Fahdil A., Sudesh K., Yousif E. Screening and evaluation of poly(3-hydroxybutyrate) with Rhodococcus. equi using different carbon sources. Arab. J. Sci. Eng. 2017;42:2371–2379. doi: 10.1007/s13369-016-2327-8. [DOI] [Google Scholar]
- 41.Ahmed D.S., El-Hiti G.A., Yousif E., Hameed A.S., Abdalla M. New eco-friendly phosphorus organic polymers as gas storage media. Polymers. 2017;9:336. doi: 10.3390/polym9080336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Curreli S., Escudero-Adán E.C., Benet-Buchholz J., Kleij A.W. A modular approach towards nonsymmetrical bis(metallosalen) building blocks. Eur. J. Inorg. Chem. 2008:2863–2873. doi: 10.1002/ejic.200800274. [DOI] [Google Scholar]
- 43.Karabocek N., Eski P., Baskan O., Karabocek S. Synthesis, characterization and biochemical activity of dinuclear Cu(II)/Ni(II)/Co(II) and tetranuclear Cu(II) complexes of Schiff base. Asian J. Chem. 2012;24:387–390. [Google Scholar]
- 44.Rabek J., Ranby B. Photodegradation., Photooxidation. and Photostabilization of Polymer. John Wiley; New York, NY, USA: 1975. [Google Scholar]
- 45.Jafari A.J., Donaldson J.D. Determination of HCl and VOC emission from thermal degradation of PVC in the absence and presence of copper, copper(II) oxide and copper(II) chloride. E-J. Chem. 2009;6:685–692. doi: 10.1155/2009/753835. [DOI] [Google Scholar]
- 46.Martinsson E., Hjertberg T., Sorvik E. Catalytic effect of hydrochloric acid dehydrochlorination of PVC. Macromolecules. 1988;21:136–141. doi: 10.1021/ma00179a028. [DOI] [Google Scholar]
- 47.Pepperl G. Molecular weight distribution of commercial PVC. J. Vinyl Addit. Technol. 2000;6:88–92. doi: 10.1002/vnl.10229. [DOI] [Google Scholar]
- 48.Skillicorn D.E., Perkins G.G.A., Slark A., Dawkins J.V. Molecular weight and solution viscosity characterization of PVC. J. Vinyl Addit. Technol. 1993;15:105–108. doi: 10.1002/vnl.730150211. [DOI] [Google Scholar]
- 49.Goldberg A.I., Hohenstein W.P., Mark H. Intrinsic viscosity-molecular weight relationship for polystyrene. J. Polym. Sci. Pol. Chem. 1947;2:503–510. doi: 10.1002/pol.1947.120020506. [DOI] [Google Scholar]
- 50.Mark J.E. Physical Properties of Polymers Handbook. Springer; New York, NY, USA: 2007. [Google Scholar]
- 51.Jellinek H.H.G. Aspects of Degradation and Stabilization of Polymers. Elsevier; New York, NY, USA: 1978. [Google Scholar]
- 52.Mori F., Koyama M., Oki Y. Studies on photodegradation of poly(vinyl chloride) (part 1) Macromol. Mater. Eng. 1977;64:89–99. [Google Scholar]
- 53.Júnior G.C., Silva A.P.S., Guinesi L.S. Synthesis, characterization and electropolymerization of a new polypyrrole iron(II) Schiff-base complex. Polyhedron. 2004;23:1953–1960. doi: 10.1016/j.poly.2004.04.029. [DOI] [Google Scholar]
- 54.Valko L., Klein E., Kovařík P., Bleha T., Šimon P. Kinetic study of thermal dehydrochlorination of poly(vinyl chloride) in the presence of oxygen: III. Statistical thermodynamic interpretation of the oxygen catalytic activity. Eur. Polym. J. 2001;37:1123–1133. doi: 10.1016/S0014-3057(00)00239-1. [DOI] [Google Scholar]
- 55.Binnig G., Quate C.F., Gerber C. Atomic-force microscope. Phys. Rev. Lett. 1986;56:930–933. doi: 10.1103/PhysRevLett.56.930. [DOI] [PubMed] [Google Scholar]
- 56.Kara F., Aksoy E., Yuksekdagd Z., Hasirci N., Aksoy S. Synthesis and surface modification of polyurethanes with chitosan forantibacterial properties. Carbohydr. Polym. 2014;112:39–47. doi: 10.1016/j.carbpol.2014.05.019. [DOI] [PubMed] [Google Scholar]
- 57.Zheng X.-G., Tang L.-H., Zhang N., Gao Q.-H., Zhang C.-F., Zhu Z.-B. Dehydrochlorination of PVC materials at high temperature. Energy Fuel. 2003;17:896–900. doi: 10.1021/ef020131g. [DOI] [Google Scholar]
- 58.Kasha M. Characterization of electronic transitions in complex molecules. Discuss. Faraday Soc. 1950;9:14–19. doi: 10.1039/df9500900014. [DOI] [Google Scholar]
- 59.Pospíšil J., Klemchuk P.P. Oxidation Inhibition in Organic Materials. Volume 1. CRC Press; Boca Raton, FL, USA: 1989. pp. 48–49. [Google Scholar]
- 60.Yousif E., Bakir E., Salimon J., Salih N. Evaluation of Schiff bases of 2,5-dimercapto-1,3,4-thiadiazole as photostabilizer for poly(methyl methacrylate) J. Saudi Chem. Soc. 2012;16:279–285. doi: 10.1016/j.jscs.2011.01.009. [DOI] [Google Scholar]