Table 1.
Structure of ring-substituted 1-hydroxynaphthalene-2-carboxanilides 1–24, calculated values of log P, electronic σ parameters of anilide (Ar) substituents and IC50 [µM] values related to photosynthetic electron transport (PET) inhibition in spinach chloroplasts in comparison with 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU) standard.
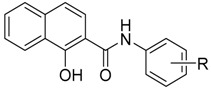
| Comp. | R | log P a | σ(Ar) a | PET Inhibition IC50 [µM] |
|---|---|---|---|---|
| 1 b | H | 4.52 | 0.60 | 31.3 |
| 2 b | 2-Cl | 5.02 | 1.05 | 29.4 |
| 3 b | 3-Cl | 5.25 | 0.85 | 7.9 |
| 4 b | 4-Cl | 5.24 | 0.75 | 10.8 |
| 5 b | 2-Br | 5.06 | 0.97 | 61.9 |
| 6 b | 3-Br | 5.39 | 0.86 | 8.2 |
| 7 b | 4-Br | 5.28 | 0.74 | 9.6 |
| 8 | 2,3-Cl | 5.76 | 1.22 | 235.3 |
| 9 | 2,4-Cl | 5.78 | 1.12 | 531.0 |
| 10 | 2,5-Cl | 5.82 | 1.22 | 59.6 |
| 11 | 2,6-Cl | 5.52 | 1.33 | 100.5 |
| 12 | 3,4-Cl | 5.99 | 1.19 | 13.3 |
| 13 | 3,5-Cl | 6.01 | 1.11 | 5.2 |
| 14 | 2,4,5-Cl | 6.31 | 1.56 | 153.2 |
| 15 | 2,4,6-Cl | 6.15 | 1.48 | 10.6 |
| 16 | 3,4,5-Cl | 6.28 | 1.46 | 8.0 |
| 17 | 2,4-Br | 5.90 | 1.11 | 505.1 |
| 18 | 2,5-Br | 5.81 | 1.23 | 7.6 |
| 19 | 2,6-Br | 5.67 | 1.33 | 78.0 |
| 20 | 2,4,6-Br | 6.34 | 1.47 | 45.0 |
| 21 | 2-Br-4-Cl | 5.80 | 1.12 | 277.5 |
| 22 | 4-Br-2-Cl | 5.88 | 1.11 | 171.0 |
| 23 | 5-Br-2-Cl | 5.82 | 1.23 | 60.3 |
| 24 | 4-Br-3-Cl | 6.02 | 1.19 | 6.7 |
| DCMU | - | - | - | 1.9 |
a calculated using ACD/Percepta ver. 2012 (Advanced Chemistry Development, Toronto, ON, Canada); b published in Gonec et al. [11].
