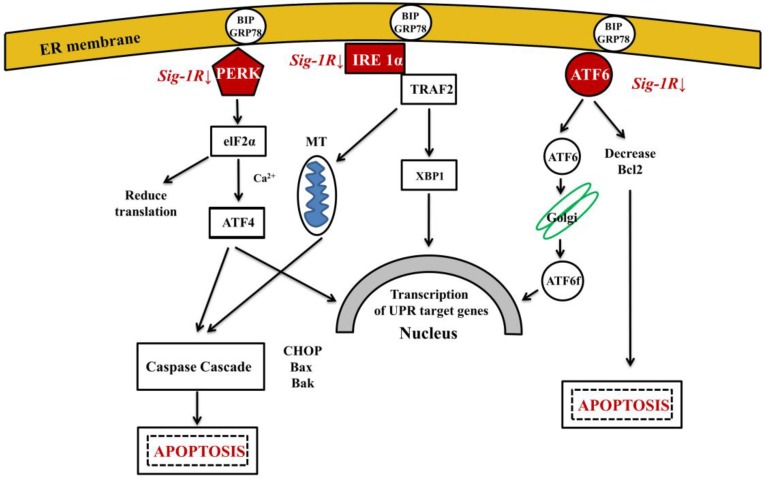
Figure 3.
Three signal pathways of ER-stress activate UPR. ER chaperone GRP78 under normal conditions binds all the three ER-stress sensors (PERK: protein kinase RNA like ER-kinase; IRE1α: inositol requiring enzyme 1α; ATF6: activating transcription factor 6). Under ER-stress GRP78 dissociates from sensors. PERK and IRE1α will be phosphorylated and oligomerized, ATF6 translocates to the Golgi (Abbreviations: eIF2α: eukaryotic translation initiation factor 2α; XBP1: X-box binding protein 1 (spliced form); TRAF2: TNF-associated factor-2; ATF4: transcriptional activator factor-4; MT: mitochondrium). ATF6, ATF4 and XBP1 activate UPR target genes to enhance the capacity of the ER to cope with unfolded proteins. Activation of Sig-1R inhibits the three branches of UPR.