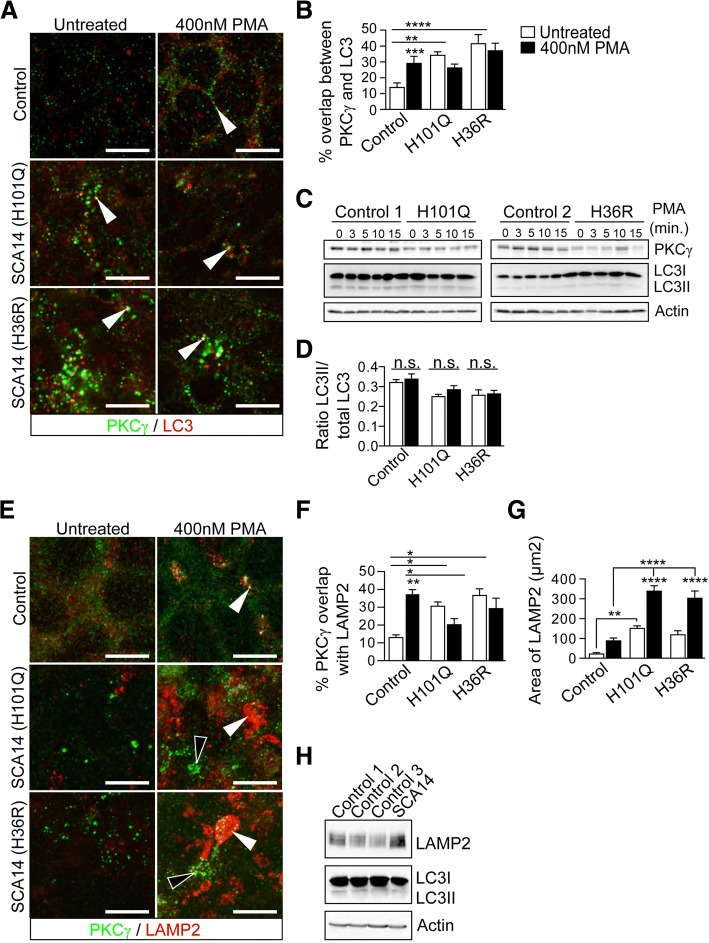
Fig. 5.
Impaired degradation of SCA14 PKCγ aggregates. a, b Control and patient iPSCs were immunostained for PKCγ and the autophagosomal marker LC3 before or after treatment with PMA for 15 min. In control iPSCs, PKCγ co-localization with LC3 (white solid arrowheads) increased upon treatment with PMA. In untreated SCA14 iPSCs, there was already a significant overlap with LC3 (white solid arrowheads), which did not further increase upon PKCγ activation (n = 3, **p < 0.01, ***p < 0.001, ****p < 0.0001, two-way ANOVA followed by Bonferroni’s post-hoc test). c Lysates of iPSCs were subjected to immunoblotting for PKCγ and LC3. LC3I represents free cytosolic cleaved LC3. LC3II represents LC3 that is anchored to the autophagosome membrane and indicates autophagosome load. d Ratio of LC3II/total LC3 levels remained constant in control and SCA14 iPSCs following PMA treatment. e, f Control and patient iPSCs were immunostained for PKCγ and the lysosomal marker LAMP2 before or after treatment with PMA for 15 min. In control iPSCs, co-localization of PKCγ with LAMP2 increased upon activation (white solid arrowheads). In SCA14 iPSCs, by contrast, lysosomes fused together into larger vesicles enclosing PKCγ aggregates (white arrowheads) in the presence of PMA. However, the majority of PKCγ aggregates did not co-localize with LAMP2-postive lysosomes (white hollow arrowheads) (n = 3, *p < 0.05, **p < 0.001, two-way ANOVA followed by Bonferroni’s post-hoc test). g The area of LAMP2 signal, representing the formation of lysosomes, significantly increased in both control and SCA14 iPSCs following PMA treatment. The lysosomal area was significantly larger in SCA14 iPSCs compared to control iPSCs (n = 3, **p < 0.01, ****p < 0.0001, two-way ANOVA followed by Bonferroni’s post-hoc test). h Cerebellar lysates were subjected to immunoblotting for LAMP2 and LC3. LC3I represents free cytosolic cleaved LC3. LC3II represents LC3 that is anchored to the autophagosome membrane and indicates autophagosome load