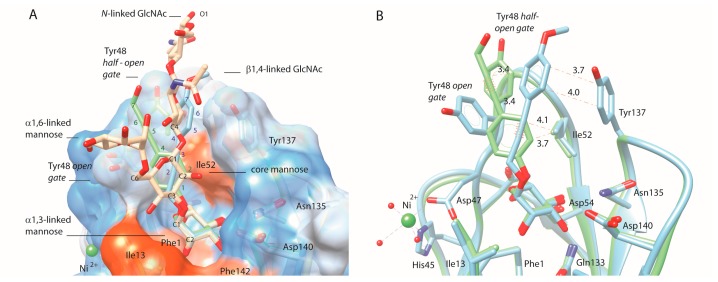
Figure 2.
Compounds that only differ in its alkyne-phenyl order as bound in the FimH crystal structures: 56 (blue, PDB entry 4av0) and its analog 61 (green, PDB entry 4auy). A wire wrapped around the bond between carbon atoms at position two and three, or five and six, respectively in compound 56 or 61, indicates the location of the alkyne. (A) Illustration of the overlay of the two ligand aglycons with the C1-C2-C3 carbon atoms of the core mannose of N-linked glycans, displayed on a hydrophobicity-colored surface presentation of the FimH lectin (dodger blue for the most hydrophilic, to white, to orange red for the most hydrophobic). The atom numbering in the aglycons starts from the first carbon following the O-glycosidic linkage; (B) Van der Waals contacts (≤4.5 Å, dashed lines in the same color as the structure) are indicating contacts made by the 56-ligand (blue) in the open tyrosine gate and by the 61-ligand (green) with the half-open conformations of Tyr48.