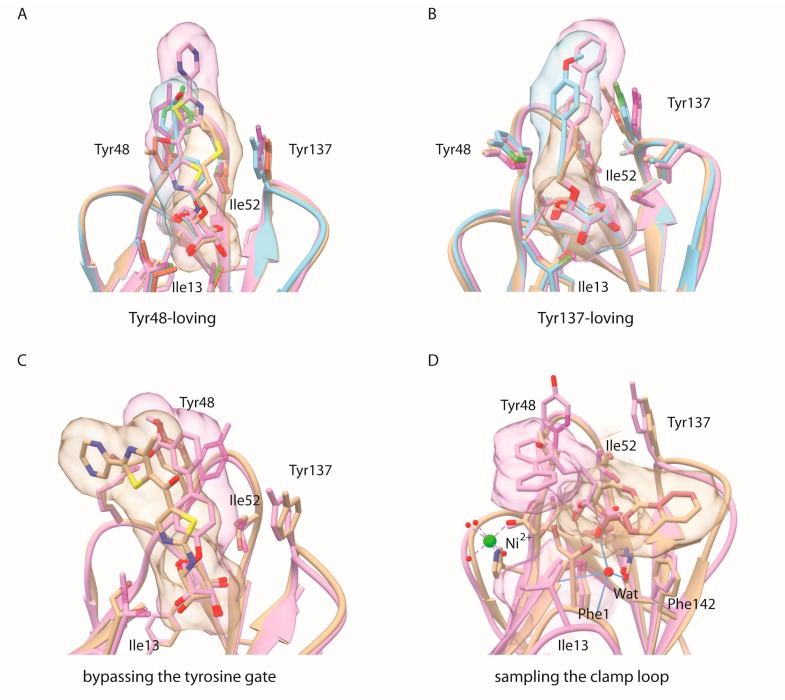
Figure 4.
Different modes of binding to FimH. The way the carbohydrate-based inhibitors interact with the FimH lectin domain can be classified in major (A,B) and two minor (C,D) binding modes (A) Tyr48-loving: PDB entries 4avh (beige), 4auy (blue) and 5mts (fuchsia); (B) Tyr137-loving: 4av5 (fuchsia), 4av0 (blue), 1tr7 (beige); (C) bypassing the tyrosine gate: the ligand para-biphenyl α-d-mannose (PBD entry 3mcy, fuchsia) and a high-scoring docking pose of the ligand in PDB entry 5mts (beige), that both stack on the other side of Tyr48, outside the tyrosine gate; (D) sampling of aromatic substituents C-linked to mannose near Ile13 of the clamp loop, that traps the internal water molecule hydrogen bonding to the axial O2 hydroxyl of mannose: PDB entry 5abz (beige) and a MD cluster (11% of the total, when the internal structural water molecule was absent at the start of the simulations) of the ortho-biphenyl α-d-mannose 117.