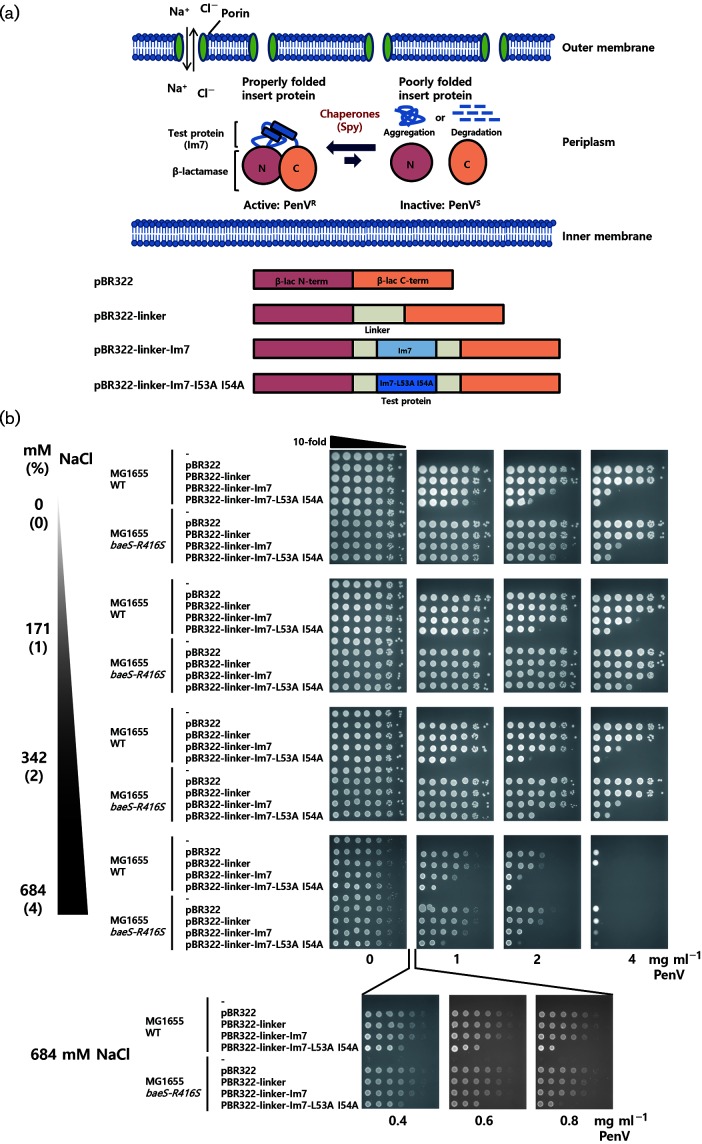
Fig. 1.
Ionic strength-dependent activity of Spy as monitored by an Im7-fused tripartite reporter system. (a) Our tripartite folding reporter consists of a test protein inserted into a permissive site within an antibiotic resistant protein. If the chaperone assists in the folding or stability of the test protein, cells harbouring this reporter will increase their antibiotic resistance. With decreased chaperone function, the tripartite protein will tend to be misfolded, aggregated, or degraded, and strains containing the reporter will show lower levels of antibiotic resistance. In the illustrated construct, β-lactamase and Im7 were used as the antibiotic-resistant protein and test protein, respectively. Im7 is integrated into a permissive site in β-lactamase using linker sequences [13]. The Im7-fused tripartite reporter system and Spy exist in the periplasm. Porins in the outer membrane allow the free diffusion of compounds smaller than ~600 Da into the periplasm. Therefore, the ionic strength in the periplasm can be adjusted by simply varying the ionic composition of the media. (b) Escherichia coli cells were transformed with pBR322-derived plasmids that contain tripartite fusion folding reporters and PenV resistance was tested by spot titre assay.