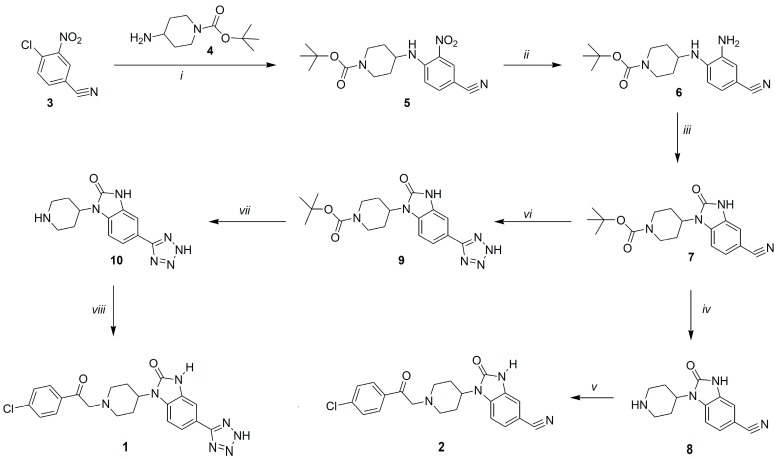
Scheme 1.
Reagents and conditions: (i) anhydr. DMF, DIPEA, microwave, 15 min., 130 °C, 5 bar; (ii) anhydr. THF, absol. EtOH, Pd-C, RT, 4 h; (iii) CDI, anhydr. THF, DMAP, microwave, 15 min., 130 °C, 7 bar; (iv) CF3COOH, CH2Cl2, RT, 2 h; (v) anhydr. DMF, Et3N, 2-Br-4’-chloroacetophenone, 0 °C → RT, 2 h; (vi) TMSN3, TBAF, 125 °C, 24 h; (vii) CF3COOH, CH2Cl2, RT, 2 h; (viii) anhydr. DMF, Et3N, 2-Br-4’-chloroacetophenone, 2 h, 0 °C → RT.