Figure 3.
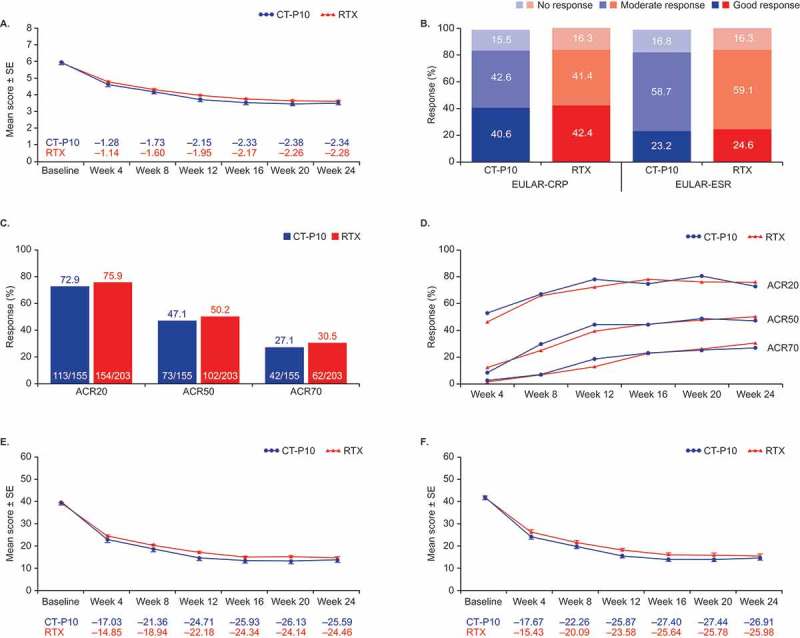
Efficacy outcomes (efficacy populationa). (A) Mean change from baselineb in DAS28-CRP. (B) Proportions of patients with good, moderate, or no EULAR response at week 24c. (C) Proportions of patients achieving clinical response at week 24 according to the ACR20, ACR50, and ACR70 criteria. (D) ACR response over time. (E) Mean change from baseline in clinical disease activity index. (F) Mean change from baseline in simplified disease activity index.
ACR = American College of Rheumatology. CI = confidence interval. CRP = C-reactive protein. DAS28 = Disease Activity Score using 28 joint counts. ESR = erythrocyte sedimentation rate. EULAR = European League Against Rheumatism. RTX = rituximab. SE = standard error. aCT-P10, N = 155; RTX, N = 203. bData shown are non-adjusted arithmetic means. cTwo patients in the CT-P10 group were non-evaluable at week 24 as they had undergone joint surgery during the study and were excluded.
