Figure 6.
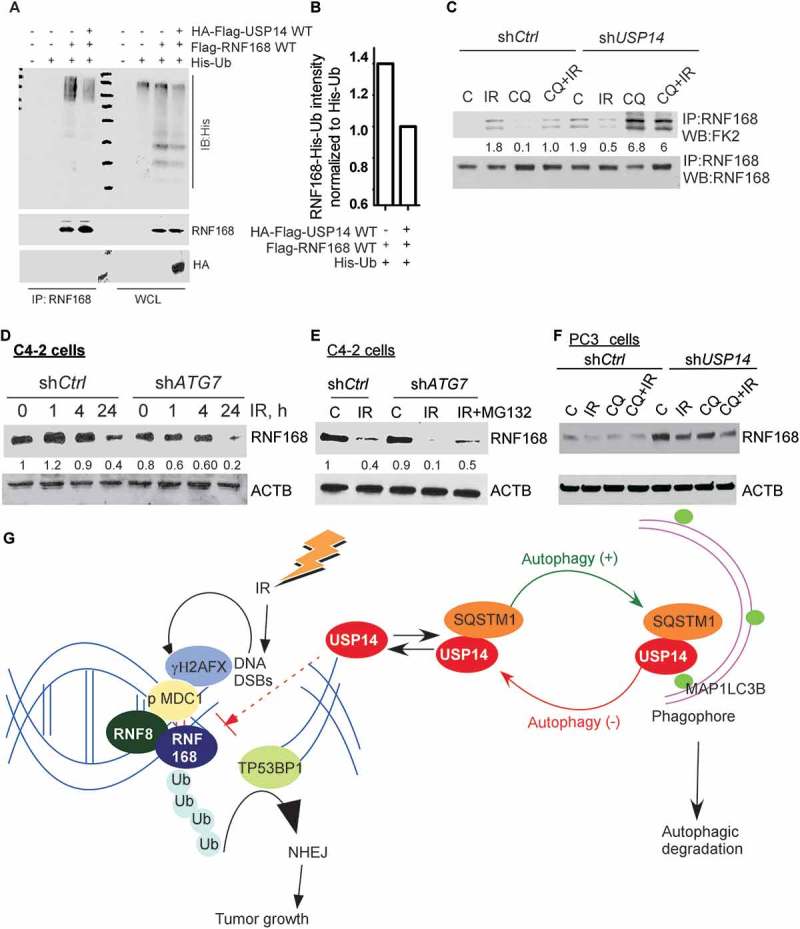
USP14 regulates the levels of RNF168 by modulating RNF168 ubiquitination. (a) 293T cells were transfected with the indicated constructs, followed by immunoprecipitation of RNF168 and immunoblotting using the indicated antibodies. The corresponding WCL were used as input controls. (b) Quantitative representation of RNF168-Ub determined in A, in the presence or absence of USP14, normalized to His-Ub levels. (c) Ub of RNF168 determined by immunoprecipitation of RNF168 in shCtrl or shUSP14 expressing C4-2 and following IR +/− CQ followed by western blotting using anti-FK2 antibody. The numbers below the blots correspond to band signal intensities compared to the untreated control in shCtrl cells. (d and e) Western blot analysis for RNF168 following the indicated treatments in C4-2 cells stably expressing shCtrl or shATG7, and (f) PC3 cells stably expressing shCtrl or shUSP14. ACTB/β-actin was used as the loading control. (g) Model for role and regulation of USP14 in autophagy-dependent DNA damage response. IR induced DNA DSBs are marked by γH2AFX followed by MDC1, which in turn recruits the RNF8-RNF168 E3 ligases to ubiquitinate (Ub) histones, leading to TP53BP1 recruitment and hence the NHEJ repair pathway that promotes tumor growth. USP14 antagonizes Ub-signaling in DDR by deubiquitinating RNF168 and inhibiting TP53BP1 recruitment. In addition, USP14 directly interacts with SQSTM1 and is targeted for autophagic degradation under normal conditions. Thus, USP14 levels are kept under check by autophagy. When autophagy is inhibited, USP14 is sequestered in SQSTM1 aggregates, and hence there is an increase in USP14 total protein levels and its recruitment to DSB sites. This, in turn, impairs DDR and, therefore, autophagy inhibition sensitizes PCa cells to IR by inhibiting DNA repair that is rescued by USP14 inhibition or depletion.
