Figure 3.
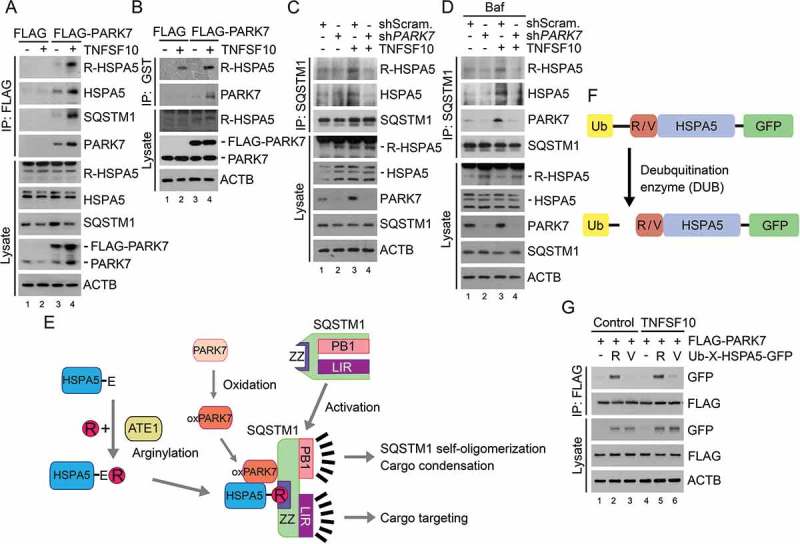
TNFSF10 induces the interaction of oxidized PARK7 with R-HSPA5. (a) HCT116 cells were transfected with a plasmid encoding FLAG or FLAG-PARK7. After 48 h, the cells were treated with 10 ng/ml TNFSF10 for 4 h. Cell lysates were immunoprecipitated with anti-FLAG antibody, followed by immunoblotting with the indicated antibodies. (b) HCT116 cells stably expressing FLAG or FLAG-PARK7 were treated with 5 ng/ml TNFSF10 for 4 h. Cell lysates were incubated with GST-PARK7 proteins for 2 h then immunoprecipitated with glutathione bead, followed by immunoblotting with the indicated antibodies. (c) HCT116 cells were treated with 10 ng/ml TNFSF10 for 4 h. Cell lysates were immunoprecipitated with anti-SQSTM1 antibody, followed by immunoblotting analysis. (d) HCT116 cells were treated with 200 nM bafilomycin A1 (Baf) for 6 h or cultured in the presence of 200 nM bafilomycin A1 for 2 h then additionally treated with 10 ng/ml TNFSF10 for 4 h. Cell lysates were immunoprecipitated with anti-SQSTM1 antibody, followed by immunoblotting analysis. (e) A schematic diagram indicating the interaction between arginylated HSPA5, oxidized PARK7 (oxPARK7), and activated SQSTM1. In this mechanism, HSPA5, PARK7 and SQSTM1 are each modified, and the modification of these 3 components constitutes the complex of 3-way interaction. (f) A schematic diagram in which recombinant Ub-R/V-HSPA5-GFP proteins are processed by a deubiquitination enzyme (DUB). In this mechanism, Ub-R/V-HSPA5-GFP is expressed in HCT116 cells and its ubiquitin is cleaved by DUB and arginine or valine is exposed as a result of cleavage as shown. (g) HCT116 cells were co-transfected with plasmids encoding FLAG-PARK7 and Ub-R-HSPA5-GFP or Ub-V- HSPA5-GFP. After 48 h, the cells were treated with 10 ng/ml TNFSF10 for 4 h. Cell lysates were immunoprecipitated with anti-FLAG antibody followed by immunoblotting with anti-GFP or anti-FLAG antibody.
