Figure 9.
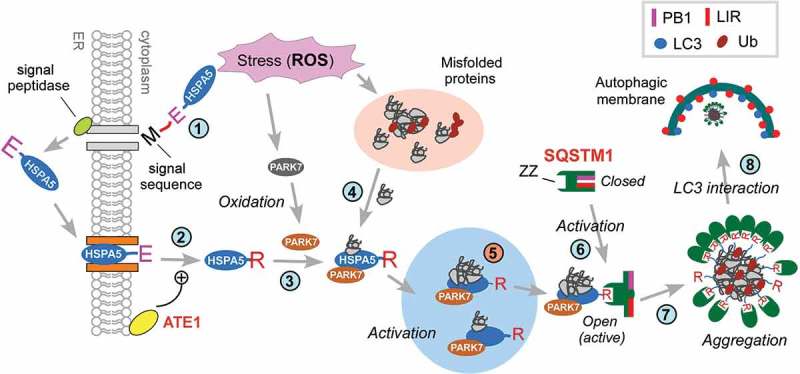
Hypothetical model for the role of PARK7 in autophagic protein quality control. In this model, TNFSF10 causes mitochondrial misregulation and oxidative stress associated with the excessive generation of ROS. This causes the formation of cytosolic misfolded proteins that are tagged with Ub but cannot be degraded by the UPS (step 1). In response to the proteotoxicity, cells induce autophagic protein quality control, which involves the Nt-arginylation of the ER-resident HSPA5 (step 2). In parallel, PARK7 is oxidized (step 3). The resulting PARK7 binds R-HSPA5 as its cofactor/co-chaperone that facilitates the ability of association with binding Ub-tagged misfolded protein clients (step 4) and enhances the ability of R-HSPA5 to activate SQSTM1 (step 5). Our earlier work [30] has shown that the Nt-Arg of R-HSPA5 binds the ZZ domain of SQSTM1 and allosterically activates the conformation of SQSTM1, exposing PB1 and LIR domains of SQSTM1 (step 6). This enables PB1-mediated self-aggregation of SQSTM1 along with R-HSPA5 and Ub-conjugated misfolded cargoes (step 7) and LIR-mediated interaction with LC3 (step 8), facilitating the autophagic removal of cytotoxic misfolded proteins and their aggregates. In this R-HSPA5-SQSTM1 circuit, PARK7 acts as a cofactor/co-chaperone of R-HSPA5 to modulate SQSTM1-dependent macroautophagy under TNFSF10-induced stresses and possibly other types of stress as well.
