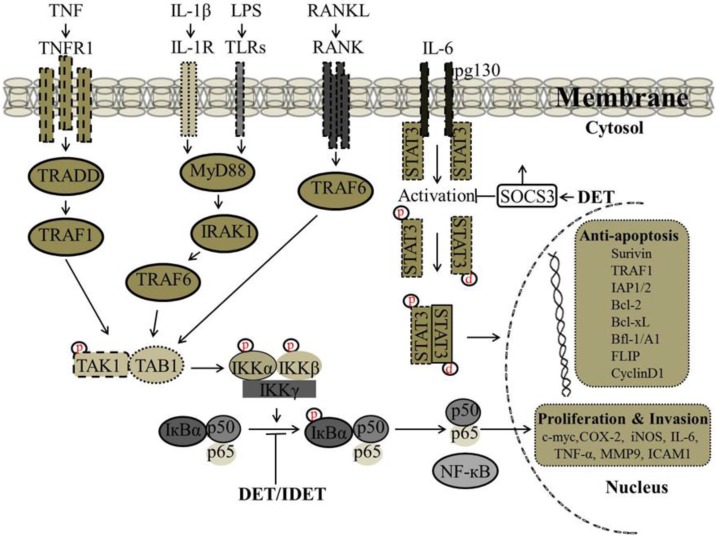
Figure 4.
Proposed mechanism by which DET and IDET inhibit nuclear factor kappa B (NF-κB) and signal transducers and activators of transcription 3 (STAT3) activation and NF-κB and STAT3-regulated gene expression involved in cell proliferation and invasion. DET and IDET inhibited the activation of NF-κB induced by various inflammatory stimuli such as (tumor necrosis factor (TNF), lipopolysaccharide (LPS), interleukin-1 beta (IL-1β) which activates NF-κB through different pathways. DET and IDET inhibited NF-κB activation at the same step of all these stimuli. Inhibition of NF-κB activation was associated with decreased phosphorylation and the degradation of IκB-α and upstream activation of IκB kinase-alpha/-beta (IKK-α/-β) and IKK kinase activities. DET and IDET down-regulated the expression of NF-κB-regulated gene products involved in invasion such as matrix metalloproteinase (MMP-9) and intercellular adhesion molecule 1 (ICAM-1); cell proliferation such as cyclooxygenase-2 (COX-2), cyclin D1, and cellular-Myc (c-Myc); and anti-apoptosis such as inhibitor of apoptosis protein-1/-2 (IAP-1/-2), B-cell lymphoma 2 (Bcl-2), B-cell lymphoma-extra-large (Bcl-xL), Bcl-2-related protein A1 also known as Bfl-1/A1, TNF receptor-associated factor (TRAF1), FADD-like IL-1beta-converting enzyme (FLICE)/caspase-8-inhibitory protein (FLIP), and survivin). DET inhibits the activation of STAT3 by reducing phosphorylation at tyrosine-705 and up-regulates the suppressor of cytokine signaling 3 (SOCS3) which is a major component for the negative regulation of the IL-6 signaling cascade; ├ Inhibition, ↑ Up-regulation.