Table 2.
Zinc chalcogenates 1–3 in the preparation of chalcogenol esters.
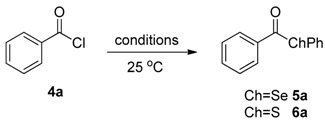
| Entry | Reagent | Medium | Time (h) | Yield (%) a | ae (%) b | Reference |
|---|---|---|---|---|---|---|
| 1 | PhSeZnCl | THF | 24 | 25 | 66 | [50] |
| 2 | PhSeZnBr | THF | 24 | 30 | 60 | [50] |
| 3 | [PhSeZnSePh] 1 | THF | 3 | 32 c | 79.6 | [56] |
| 4 | [PhSeZnSePh] 1 | THF | 3 | 40 d | 79.6 | – |
| 5 | PhSZnBr | THF | 24 | 86 | 54.6 | [57] |
| 6 | [PhSeZnSePh/PhSeH] | HClacq/Et2O | 4 | 38 | – | [56] |
| 7 | PhSeZnCl | H2O | 3 | 60 | 66 | [50] |
| 8 | PhSeZnBr | H2O | 3 | 70 | 60 | [50] |
| 9 | PhSZnBr | H2O | 3 | 65 | 54.6 | [57] |
| 10 | [PhSeZnSePh] 1 | H2O | 0.5 | 83 | 79.6 | – |
| 11 | [PhSeZnSePh]TMEDA 3 | H2O | 0.5 | 66 | 77 | – |
| 12 | [PhSZnSPh] 2 | H2O | 0.5 | 50 | 76 | – |
a Conversion estimated by NMR; b Atom economy = m.w. of final product × 100/Σ (m.w. reactants); c 1 was prepared in the presence of 10 mol % of TfOH. Compound 5a was formed together with 34% PhC(O)O(CH2)4SePh and 28% PhC(O)O(CH2)4 O(CH2)4SePh; d 1 was prepared in the presence of 10 mol % of TFA. Compound 5a was formed together with 27% PhC(O)O(CH2)4SePh and 5% PhC(O)O(CH2)4 Cl.
