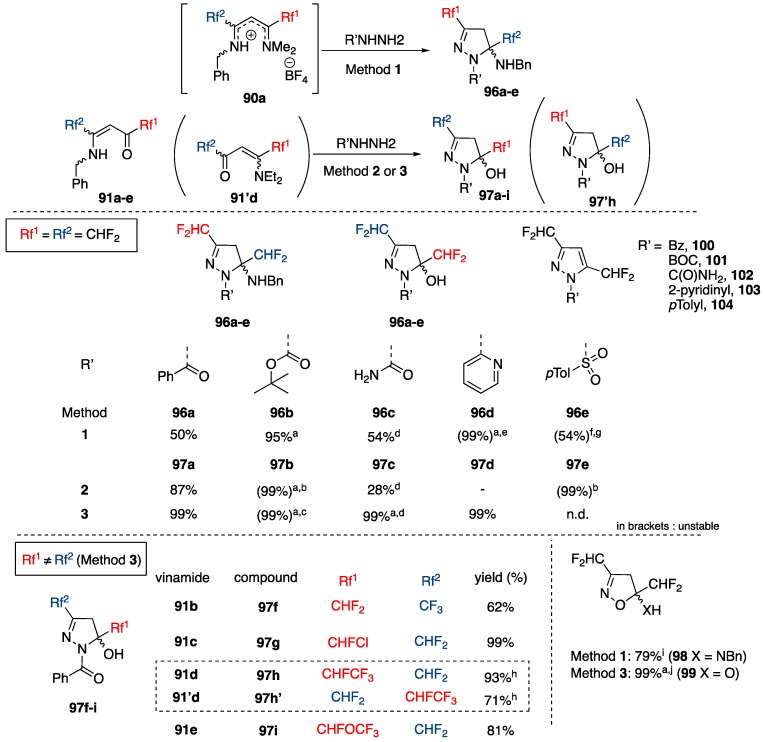
Scheme 28.
Regioselective preparation of 5-N-benzylamino- and 5-hydroxypyrazolines and isoxazolines. Method 1: hydrazine, conc. H2SO4, MeCN, 25–50 °C, 1 h. Method 2: hydrazine, toluene/MeCN, 120–140 °C, MW, 0.5–2 h. Method 3: hydrazine, HFIP (hexafluoropropan-2-ol), 100–140 °C, 0.5–5 h. a 19F NMR yield with PhF as internal standard. b R group cleaved between 120 °C and 150 °C. bis(CHF2)-NH-pyrazole 71 formed. c R group cleaved between 80 °C and 120 °C. bis(CHF2)-NH-pyrazole 71 formed. d prepared from a mixture of semicarbazide hydrochloride and NEt3, with no acid added. e Pyrazole 103 was isolated directly. f No conc. H2SO4 used. g N-(pTolyl)-pyrazole (104) was separated by chromatography from pyrazoline 96e (29% isolated). h Pyrazolines 97h and 97’h were prepared from a 65/35 mixture of vinamides 91d and 91’d and separated by chromatography. i Hydroxylamine (50 wt. % aq.) used instead of hydrazine. j Hydroxylamine·HCl used instead hydrazine.