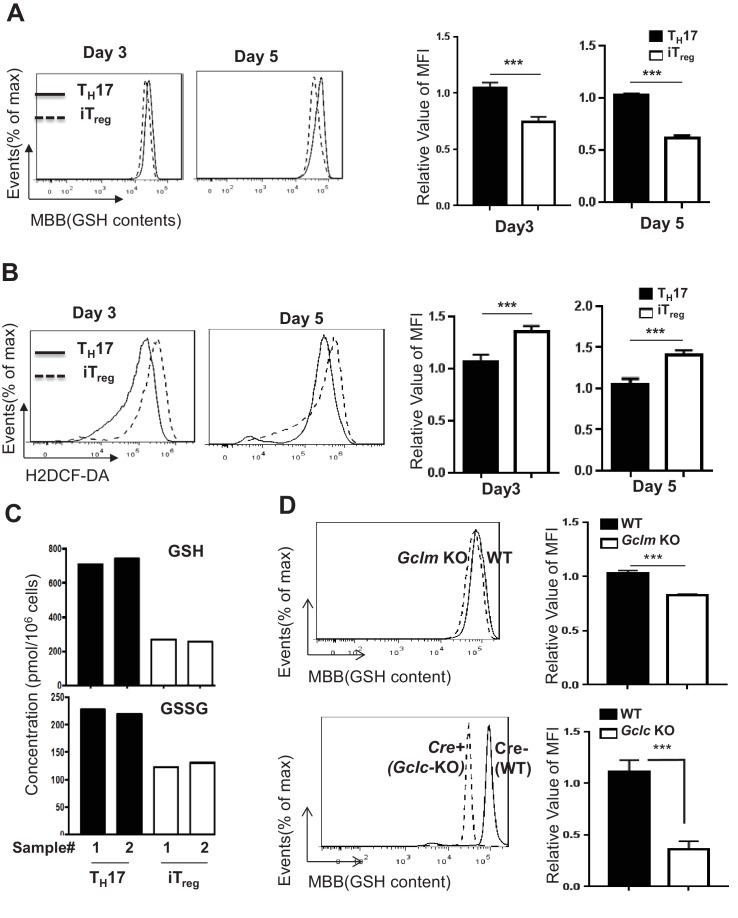
Figure 4. TH17 cells preferentially maintain higher level of GSH than iTreg cells.
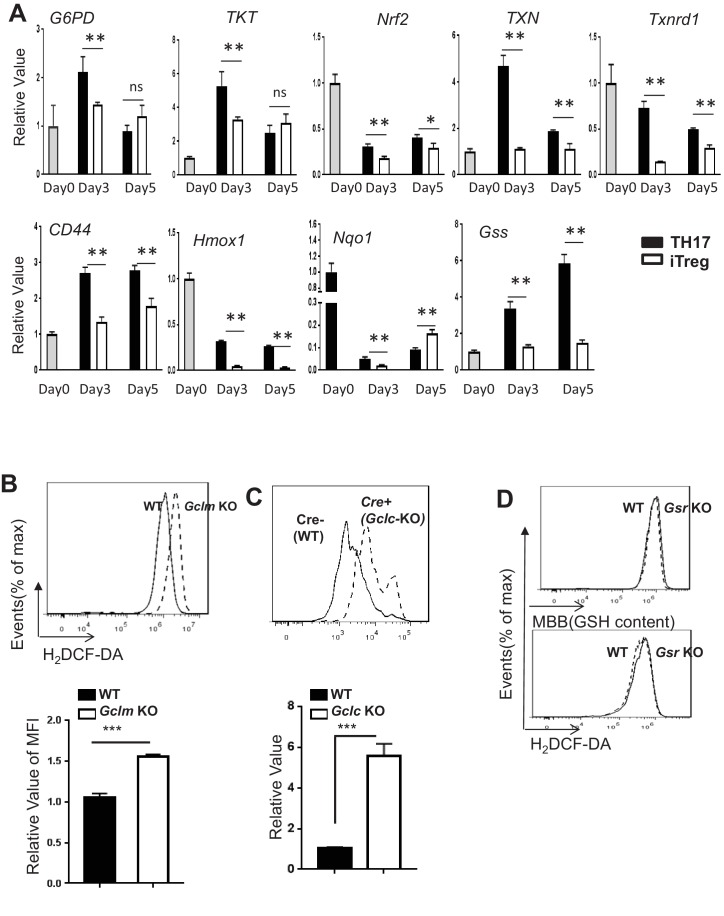
(A–B) Naive CD4+ T cells from C57BL/6 mice were differentiated under iTreg or TH17–inducing conditions and cells were collected at indicated times, followed by measuring intracellular GSH (A) and ROS (B) by FACS. (C) Naive CD4+ T cells from C57BL/6 mice were differentiated under TH17 or iTreg–inducing conditions for 5 days. The intracellular levels of GSH and GSSG were determined by mass spectrometry. (D) Naive CD4+T cells from WT and Gclm KO (top) or WT (CD4-Cre-, Gclcfl/fl) and Gclc KO (CD4-Cre+, Gclcfl/fl, (bottom) were differentiated under TH17-inducing conditions for 5 days, followed by the measurement of GSH levels. Data in Figure 4A–D are representative of three independent experiments. Data represent the mean ± S.D.