Figure 2. Clinical validity of APOL1 testing for disease diagnosis and prognosis in African Americans.
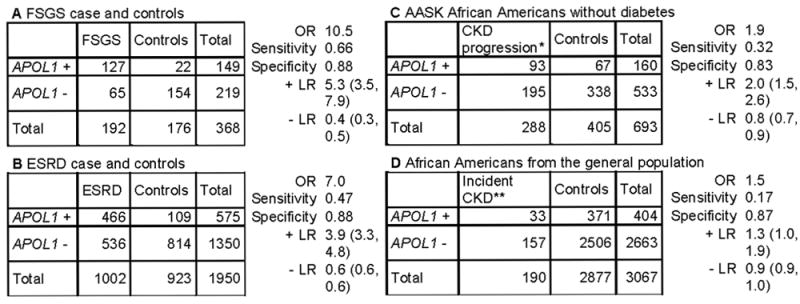
We estimated the sensitivity, specificity, positive likelihood ratio (+LR, true positive/false positive) and negative likelihood ratio (-LR, true negative/false negative) for APOL1 testing (and 95% confidence intervals) using published data. The settings designated as A and B are related to FSGS and ESRD diagnosis, respectively. C and D are genetic testing used for prognosis. The +LR is moderate to low when a genetic testing is performed for diagnosis of APOL1-related FSGS (A) and ESRD (B), respectively, and so a positive test may be useful only for diagnosis of APOL1-related FSGS. The -LR is close to zero in setting A, so a negative test could help to rule out APOL1-related FSGS. When testing African Americans with non-diabetic CKD (C) or screening African Americans without CKD in the general population (D), the +LR is low, supporting the low yield for an APOL1 genetic testing used for prognosis or screening. In addition, a negative test is not helpful to rule out future disease or counseling in settings C and D since the -LR is close to 1. Data obtained from table 1 in Genovese et al12 (A & B), table 2 in Parsa et al15 (C) and table 2 in Foster et al 16 (D). APOL1 + is high-risk genotypes and APOL1 – is low risk genotypes. We assumed the disease is causally related to APOL1 high-risk genotypes, although other genetic variants could contribute to CKD. *incident ESRD or doubling of serum creatinine **An eGFR < 60 ml/min/1.73 m2 at follow-up
