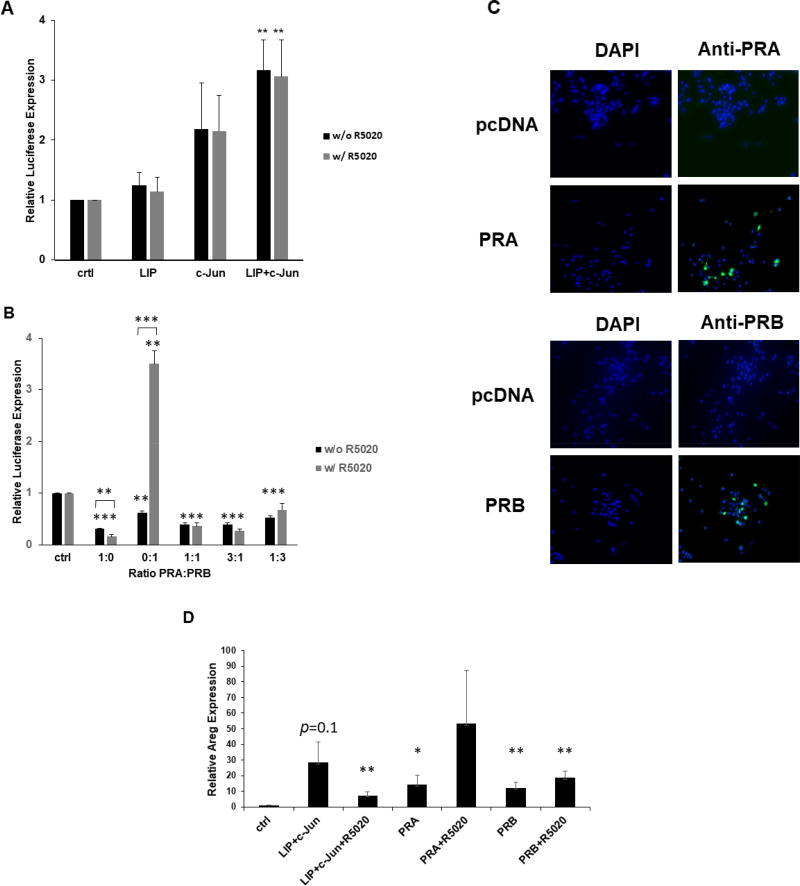
Fig. 4.
Co-expression of LIP and c-Jun increases 2×PRE promoter activity independently of R5020. (A) Transient co-transfection of C/EBPβ and c-Jun expression vectors were carried out in duplicate and repeated at least three times in MC7-L1 cells with the 2×PRE promoter-reporter. Values were normalized and statistics performed as described in Fig. 2. **, p < 0.05. (B) Transient co-transfection of PRA and PRB expression vectors, either singly or in various ratios as indicated, were carried out in duplicate or repeated at least three times in MC7-L1 cells with the 2×PRE promoter-reporter. Values were normalized and statistics performed as described in Fig. 2. **, p < 0.05; ***, p < 0.01. (C) Immunofluorescent detection of PRA and PRB in respectively transfected MC7-L1 cells. Transfection with empty pcDNA3.1 (pcDNA) vector served as a control. Left panels display DAPI (blue) staining. Right panels display PRA and PRB immunofluorescence (green) superimposed on the DAPI staining. (D) Transient co-transfection of 1 µg C/EBPβ and 1 µg c-Jun expression vectors, and 2 µg PRA and PRB expression vectors per 5×105 cells were carried out in duplicate and repeated three times in MC7-L1 cells. Transfected cultures were treated with and without R5020. Amphiregulin (Areg) and GAPDH RNA expression were quantitated by qRT-PCR. Amphiregulin values were normalized to GAPDH levels, and then normalized to a relative value of 1.0 for “ctrl” cells receiving only “empty” pcDNA3.1. *, p<0.1; **, p<0.05 for comparisons to “ctrl”.