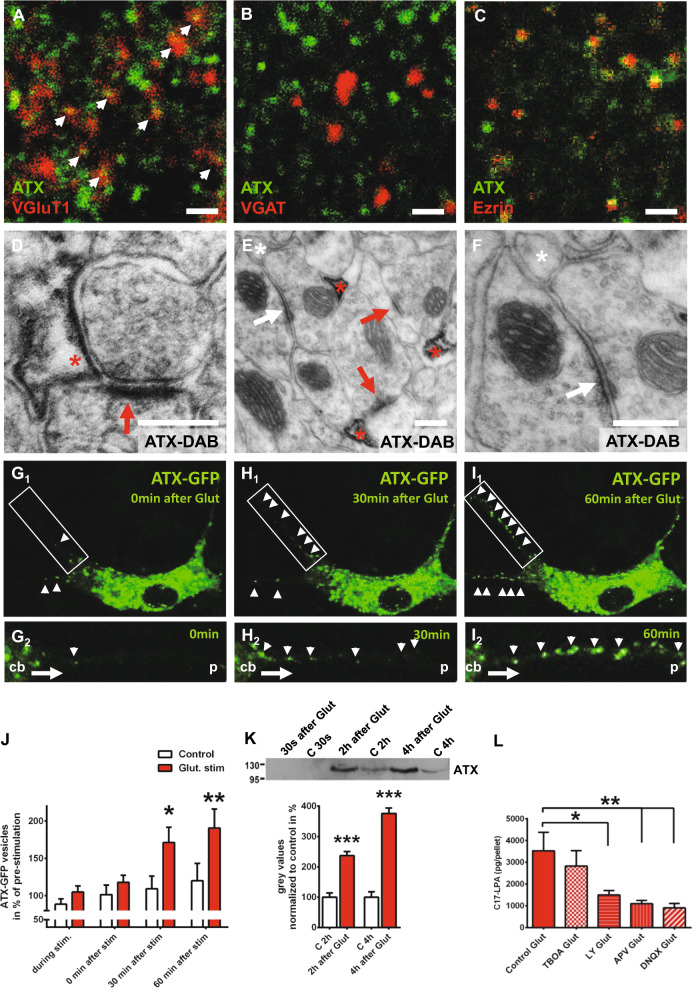
Fig. 1.
ATX is expressed by perisynaptic astrocytic processes (PAPs) at excitatory synapses and is regulated by glutamate. a, b ATX shows abundant colocalization with VGluT1, a marker of excitatory synapses, but not with VGAT, a marker of inhibitory synapses. c ATX is expressed at PAPs as shown by colocalization with Ezrin, a specific marker for PAPs. Scale bars a–c: 1 µm. d Electron microscopic image showing ATX expression (displayed by DAB-precipitation [red asterisk] at the astrocytic membrane close to the synapse) on PAP membranes surrounding glutamatergic synapses. Dense membrane in the postsynaptic compartment (red arrow) represents the postsynaptic density (PSD) of glutamatergic synapses (see also Fig. S1B). e Immune electron microscopic analysis revealed no ATX expression in astrocytic processes (white asterisk) at inhibitory synapses (white arrow). In contrast, ATX expression (shown by DAB precipitation) was prominent in astrocytic processes (red asterisk) at excitatory synapses (red arrow, see also Fig. S1C,D). f Higher magnification showing the inhibitory synapse (white arrow) and the adjacent ATX-negative astrocytic process (white asterisk). Scale bars d–f: 200 nm. G1-I1 Live imaging of astrocytes transfected with an ATX-GFP-expressing construct shows a clear increase of ATX-GFP-positive vesicles transported along their processes towards the periphery after glutamate stimulation (500 µM for 15 min). G2-I2 Live-imaging pictures at higher magnification show transport of ATX-GFP-positive vesicles (white arrow heads) along an astrocytic process from the cell body (cb) toward the periphery (p). In contrast, ATX-GFP-positive vesicles were rarely seen in processes of control, non-stimulated astrocytes (Fig. S1E). j Quantitative analysis of ATX-GFP vesicles in astrocytic processes, during glutamate stimulation and at 0, 30 and 60 min after glutamate stimulation (n = 13 control astrocytes and 14 glutamate-stimulated astrocytes; two-way RM ANOVA with Bonferroni post hoc). k ATX secretion in the astrocytic culture supernatant was significantly increased upon glutamate stimulation (500 µM Glut stimulation for 15 min) when compared with supernatant from control (c), non-stimulated astrocytic cultures as shown by western blot (n = 5 for 2 h values and n = 4 for 4 h values, two-tailed t-test). l C17-LPA synthesis in the astrocytic compartment was significantly decreased upon application of specific glutamate receptor inhibitors (50 µM LY367385 for mGluR1a, 50 µM APV for NMDA-Rs and 10 µM DNQX for AMPA-Rs), whereas 40 µM DL-TBOA, an inhibitor of astrocytic glutamate transporters, did not affect astrocytic C17-LPA levels as shown by mass spectrometry analyses (n = 6 experiments per group, one-way RM ANOVA with Bonferroni post hoc). Bars represent mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001