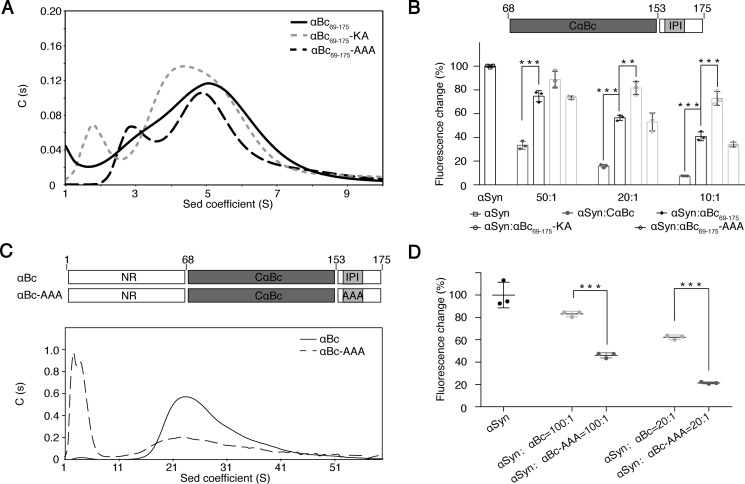
Figure 5.
Influence of the IPI-β4/β8 interaction in αBc multimerization and chaperone activity. A, sedimentation velocity analysis of αBc(69–175), αBc(69–175)-KA, and αBc(69–175)-AAA at 20 °C at a concentration of 5 mg/ml. B, comparison of the chaperone activity of CαBc, αBc(69–175), αBc(69–175)-KA, and αBc(69–175)-AAA for preventing αSyn aggregation. The ThT value was taken at the 58-h time point from the ThT kinetics curves. Error bars correspond to mean ± S.E. with n = 3. *** indicates p < 0.005, and ** indicates p < 0.01. C, sedimentation (Sed) velocity analysis of αBc (0.7 mg/ml) and αBc-AAA (0.7 mg/ml) at 20 °C. D, comparison of the chaperone activities of αBc and αBc-AAA for inhibiting αSyn aggregation. Error bars correspond to mean ± S.E. with n = 3. *** indicates p < 0.005.