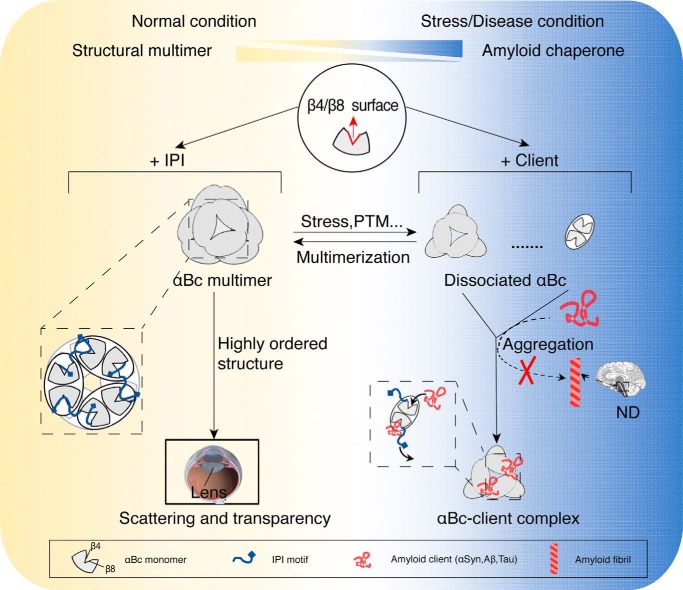
Figure 6.
Schematic diagram of the regulation of αBc for chaperone activity and multimerization. Under normal conditions, αBc forms polydisperse multimers (left) with limited chaperone activity in which the β4/β8 surface is occupied by the neighboring IPI peptide. In lens, multimerization enables αBc to act as a structural protein that packs into higher-order structures to maintain the scattering and transparency of lens. Under stress or disease conditions, αBc disassembles to small multimers (e.g. dimers and hexamers) in response to different stimuli, e.g. stress or phosphorylation (PTM), and exhibits much enhanced chaperone activity. The activated αBc (right) may capture different pathological amyloid clients (e.g. αSyn, Aβ, and Tau) with a more exposed β4/β8 surface and prevent them from forming irreversible amyloid aggregations, which are closely associated with a variety of neurodegenerative diseases (ND). The regulation of αBc between these two functions is accomplished by the competitive binding of the IPI motif and amyloid clients to the key β4/β8 surface of αBc.