Figure 4.
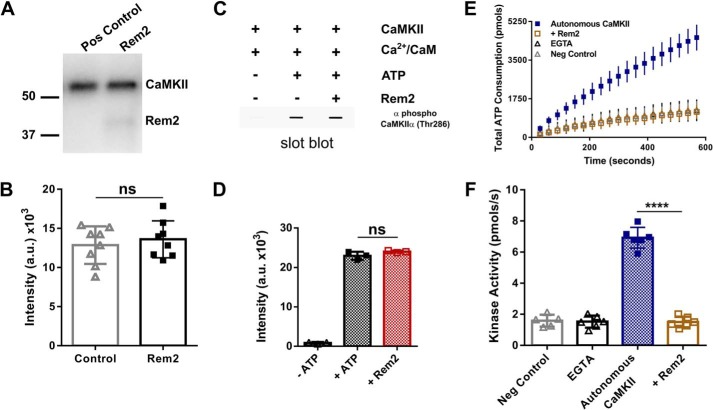
Rem2 inhibits autonomous CaMKIIα but not CaMKII autophosphorylation at Thr-286. A, autophosphorylation of CaMKIIα (80 nm) was induced by incubation of the enzyme with Ca2+/CaM (1 mm/3.33 μm) and [γ-32P]ATP (100 μm) in the absence or presence of 500 nm Rem2 for 2 min. The reaction mixture was separated by SDS-PAGE, the gel was dried, and visualization and quantification of 32P-labeled proteins were obtained using a phosphorimaging system. A representative experiment is shown. The numbers represent the position of protein molecular mass standards in kDa. B, quantification of all CaMKIIα autophosphorylation experiments as in A (n = 8; standard deviations are shown; ns, not significantly different (p = 0.7768), Wilcoxon–Mann–Whitney test). C, representative experiment of slot-blot analysis of CaMKIIα using an antibody specific for phospho-Thr-286. CaMKIIα was incubated for 2 min with the reagents indicated above the blot. D, quantification of all experiments as in C (n = 9; standard deviations are shown; ns, not significantly different, one-way ANOVA with Tukey's post hoc test). E, CaMKIIα (60 nm) was preincubated with ATP (500 μm) and Ca2+/CaM (1 mm/10 μm) for 2 min at room temperature to promote CaMKIIα autophosphorylation and autonomous activity. The preincubated mixture was then exposed to syntide-2 (200 μm) ([Ca2+] < 50 nm). The total ATP consumption as a function of time in the absence (blue squares) or presence of Rem2 (500 nm; gold squares) is shown. Addition of EGTA during the preincubation was used to confirm that only the autonomous activity was assayed (black triangles). A negative control (gray diamonds) was conducted in the absence of CaMKIIα. F, the kinase activity of CaMKIIα in each of the conditions described in E is shown using the same color code (n = 6; error bars depict standard deviations; ****, p < 0.0001, one-way ANOVA with Tukey's post hoc test). Pos, positive; Neg, negative; a.u., arbitrary units.