Figure 7.
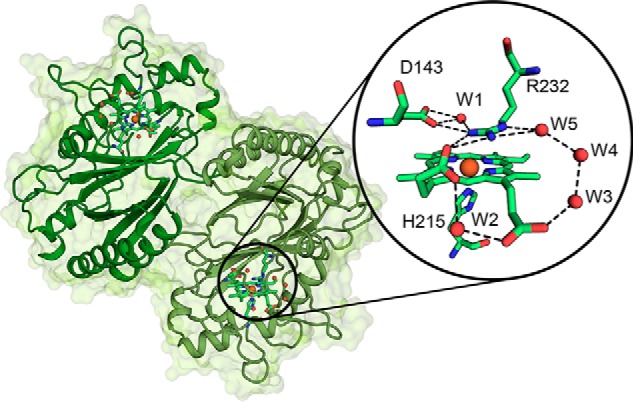
Overall and active-site crystal structure of WT KpDyP. The dimeric structure of KpDyP is shown as a cartoon (green). Heme prosthetic groups are depicted as sticks, and the heme iron is shown as an orange sphere. The round inset shows a detailed view of the active-site amino acid residues Asp-143 and Arg-232 (distal) and His-215 (proximal) and the heme b moiety as a stick representation. Hydrogen-bonding networks involving the distal residues and the heme b propionates are shown as dashed lines, and water molecules (W) and heme iron are depicted as red and orange spheres, respectively.
