Figure 8.
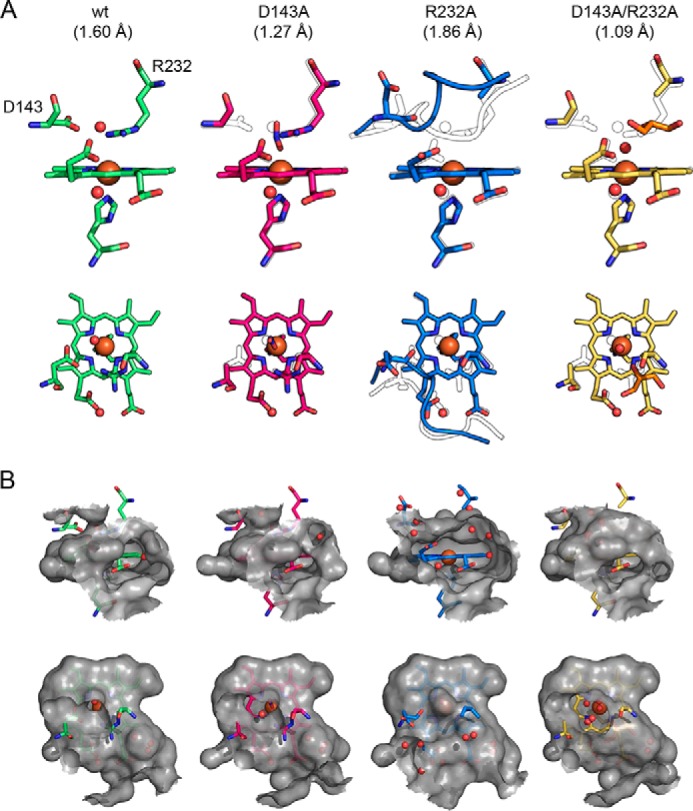
Comparison of the active-site architecture of WT KpDyP and the variants D143A, R232A, and D143A/R232A. A, representation of the active-site residues Asp-143, Arg-232, and His-215 and the heme b moiety shown from the front (first row) and top (second row). Relevant water molecules are shown as red spheres, nitrite (D143A structure) is shown in blue, and glycerol (D132A/R232A structure) is shown in orange. In all mutant structures, the WT structure is depicted as a black outline for comparison. Additionally, the backbones of residues 141–148 are shown in the R232A structure together with the respective WT conformation (black outline). B, surface of the active site and access channels shown in a 6-Å radius from the heme iron colored in gray (semitransparent), shown from the front (first row) to visualize the surface-accessible propionates and from the top (second row) to show the distal access channel.
