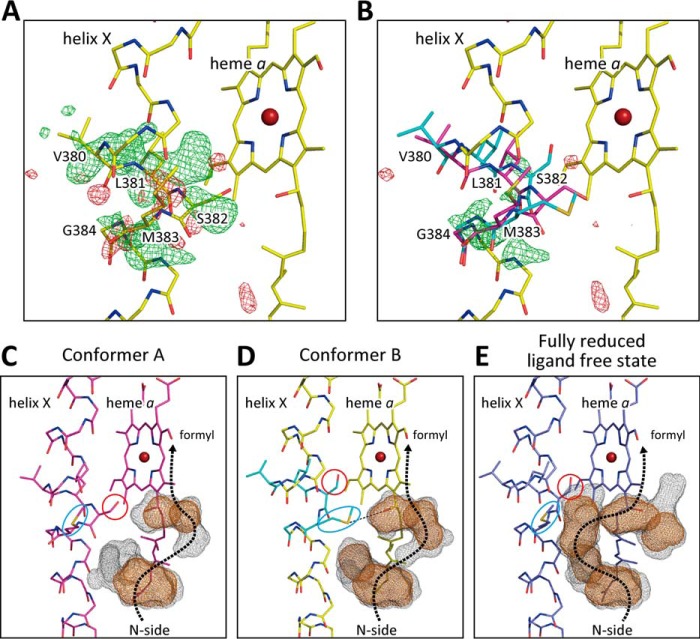
Figure 5.
Fo − Fc maps of a segment (from Val380 to Gly384) of helix X. The residual electron densities are indicated with green mesh (+3.0 σ) and red mesh (−3.0 σ). A, determined assuming the conformation of the resting oxidized state (yellow). B, determined assuming the two conformations, one closely similar to that of the resting oxidized state (magenta) (conformer A) and the other obtained with 20 mm azide (light blue) (conformer B), in 1:1 occupancy ratio. C and D, water cavities located near the P-side end of the water channel in conformers A and B, detectable at 20 mm azide, respectively. The brown- and gray-dotted surfaces are determined by the program VOIDOO (39) using probes with a radius of 1.2 and 0.8 Å, respectively. E, cavities of the open conformation of the fully reduced ligand-free state, detectable in areas corresponding to those of C and D. The same color code as C and D was used to indicate the water-accessible surfaces. The dotted arrows denote possible locations of water channels. The locations of side chains of Ser382 and Met383 are indicated by red circles and blue ovals, respectively. The hydrogen bond between Met383 and the OH group of the hydroxy farnesyl ethyl group of heme a is indicated by a dotted line.