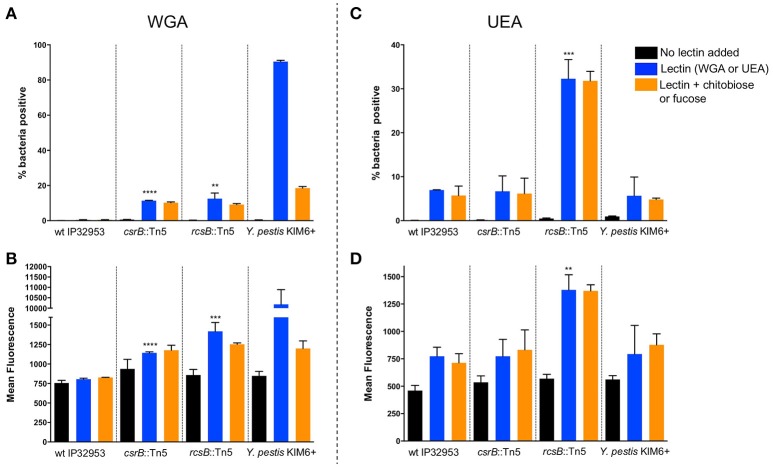
Figure 4.
Loss of rcsB and csrB alters lectin binding to bacterial surfaces. Bacteria were incubated with FITC-labeled WGA (A,B) or UEA (C,D) lectins. Flow cytometry was used to measure the proportion of bacteria with any detectable binding (A,C) or the mean fluorescence of the population (B,D). For each strain that was tested, a control with no lectin was included to establish baseline fluorescence, as well as samples with competing sugars (chitobiose or fucose). Results are means and standard deviations for a representative experiment that was performed four times. To see if the csrB::Tn5 or rcsB::Tn5 mutants bound lectin differently than the wild type strain, one-way ANOVA analysis was performed with Tukey's correction for multiple comparisons using the lectin-treated samples (blue bars). The csrB::Tn5 and rcsB::Tn5 mutants bound significantly more WGA than the wild type strain, but only the rcsB::Tn5 mutant bound more UEA than wild type. Y. pestis binding was tested as a control, and it bound to WGA far more efficiently than Yptb (**p < 0.01, ***p < 0.001, ****p < 0.0001).