Figure 4.
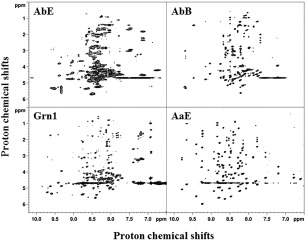
Two‐dimensional NMR TOCSY spectra showing relatively well‐resolved NH‐to‐sidechain proton connectivies of four GEM peptides, AbE, AbB, Grn1, and AaE. The TOCSY spectra are arranged in the order of increasing number of peaks and increasing resonance dispersion, for example, from 6.5 to 10 ppm for AaE covering >3.5 ppm for the NH proton resonances. Broader spectra features are also evident in the TOCSY spectra of AbE, as already seen in the one‐dimensional proton NMR spectrum of this GEM peptide (Fig. 3), which indicates either conformational flexibility and/or sample aggregation.
