Figure 6.
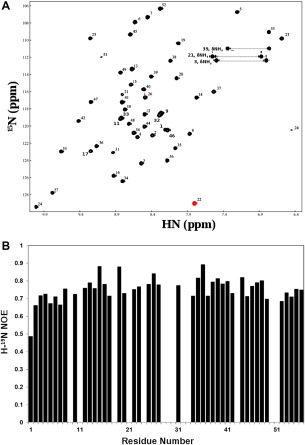
Heteronuclear (H‐15N) NMR data showing a uniquely folded three‐dimensional structure for the GEM peptide AaE. (A) Proton (H)‐nitrogen (15N) correlation (HSQC) spectra of 15N‐labeled AaE (see the Experimental Section for sample conditions). A widespread dispersion of HSQC peaks mirrors similar characteristics of the TOCSY spectrum for AaE (Fig. 4). The HSQC peaks also have rather uniform intensities, which further indicates good folding and conformational rigidity for the three‐dimensional structure of AaE. (B) Heteronuclear (H‐15N) NOEs of AaE displaying uniformity, with the exception of terminal residue Asp1, of the atomic motions in AaE, again indicating stable folding for AaE (see text descriptions for details). Proline residues, that is, Pro18, Pro28, Pro30, Pro43, and Pro50, do not have amide protons for H‐15N NOE measurements, and heteronuclear NOE data were not quantitated for residues Ser9, His11, Ser29, Val33, Ala32, and Gly51 due to resonance overlaps and for Gln21, Trp24, and Gly51 because of diminished intensities of their H‐15N HSQC peaks.
