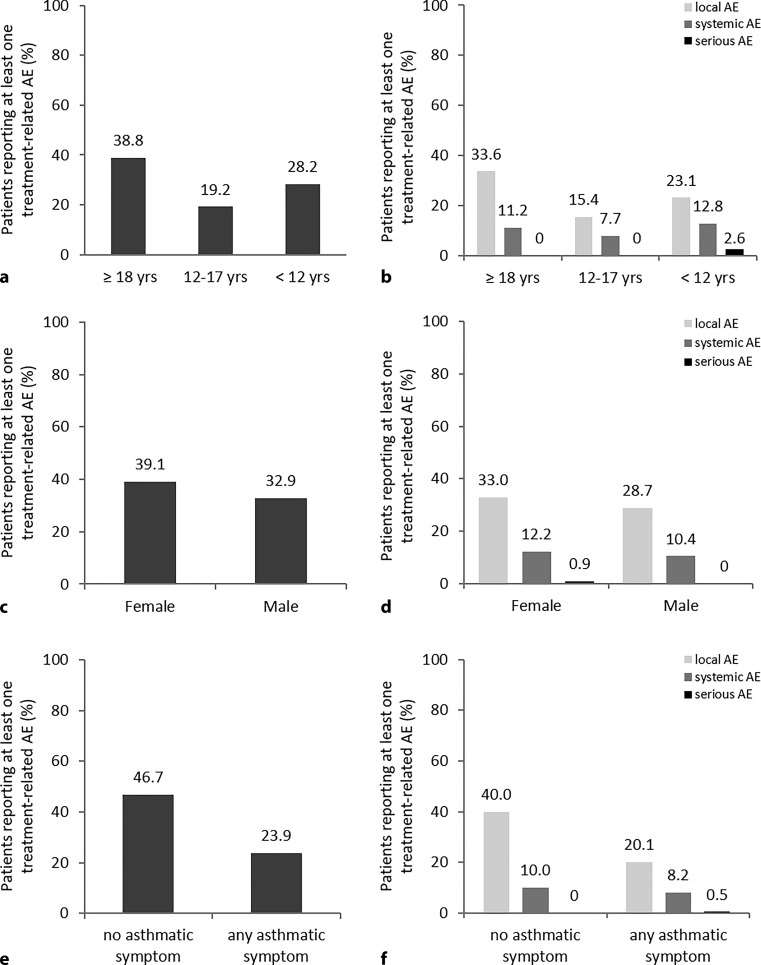
Fig. 2.
Percentage of patients reporting treatment-related adverse events related to study drug analyzed in different subgroups: age (a, b), gender (c, d), asthma status (e, f). a Data are shown for HDM-DBPC (≥18 years) and HDM-RCT(UC) (12–17 years and <12 years). b Distribution according to local, systemic and serious AE. c Percentage of female and male patients in HDM-All reporting a treatment-related AE. d Distribution according to local, systemic and serious AE. e Percentage of patients with and without asthma reporting a treatment-related AE and f Distribution according to local, systemic and serious AE. (Age (a, b): ≥18 years n = 214, 12–17 years n = 26, <12 years n = 39; Gender (c, d): female n = 115, male n = 164; Asthma status (e, f): no asthma symptom n = 60, any asthma symptom n = 184). HDM-DBPC high-dose HDM allergoid AIT double-blind placebo controlled, HDM-RCT(UC) high-dose HDM allergoid AIT usual care controlled, HDM-ALL HDM-DBPC plus HDM-RCT(UC), AE adverse event, yrs years