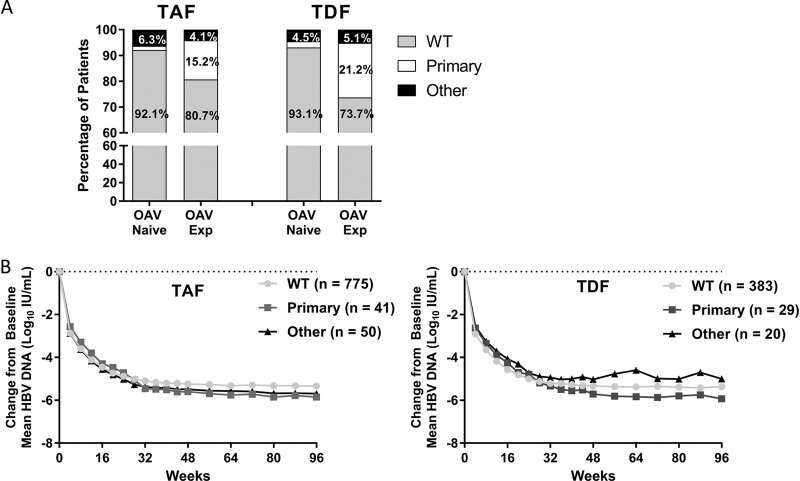
FIG 3.
Baseline resistance surveillance and impact on treatment outcomes. (A) The percentage of patients with wild-type HBV (no resistance mutations detected at baseline), primary mutations, or other mutations (defined in methods) detected at baseline by INNO-LiPA in the TAF or TDF treatment group is depicted as a percentage of the group by baseline oral antiviral experience. (B) The mean change of HBV DNA from baseline for patients in the TAF (left) or TDF (right) treatment groups is shown for patients as determined by baseline resistance analysis.