Abstract
Development of truly useful wearable physiologic monitoring devices for use in diabetes management is still in its infancy. From wearable activity monitors such as fitness trackers and smart watches to contact lenses measuring glucose levels in tears, we are just at the threshold of their coming use in medicine. Ultimately, such devices could help to improve the performance of sense-and-respond insulin pumps, illuminate the impact of physical activity on blood glucose levels, and improve patient safety. This is a summary of our experience attempting to use such devices to enhance continuous glucose monitoring–augmented insulin pump therapy. We discuss the current status and present difficulties with available devices, and review the potential for future use.
Keywords: physiological sensors, fitness trackers, wearables, diabetes management
Continuous glucose monitoring (CGM) is now accepted as standard practice for intensive glucose control in persons with type 1 diabetes (T1DM). Initially, CGM data were collected and reviewed retrospectively to assess patient daily glucose patterns, improve glucose control, and detect asymptomatic hypoglycemia. The technology then advanced to also include real-time CGM, which enabled improved patient self-management,1 the development of low glucose threshold suspend in pumps to alert/prevent hypoglycemia, and a sense-and-respond hybrid artificial pancreas (AP) insulin pump. Prototypical AP pumps using CGM data are currently in development and nearing clinical use.2
However, there are still significant limitations to the current sense-and-respond technology, including the delay between the detection of glucose levels in the interstitial fluid compared to venous levels and the delayed onset of action of current insulin analogs. Glucose levels in interstitial fluid lag behind venous levels by from 5 to 10 minutes,3 while rapid-acting insulins start to work in from 10 to 30 minutes.4 These delays impede the timeliness with which we can decrease or suspend insulin infusion with decreasing glucose levels or respond when glucose levels rise following meals. So, it is not surprising that diabetes technology researchers would explore how additional types of sensors, monitoring changes in other physiologic parameters, might contribute to pump controller algorithms and help compensate for the current issues with sense-and-respond insulin pumps. For example, Stenerson et al demonstrated how accelerometers could benefit the predictive low glucose suspend algorithm for patients with T1DM during exercise,5 while Schechter et al found that nocturnal hypoglycemia could be detected in hospitalized adolescents with T1DM using a suite of noninvasive physiological sensors.6 Breton et al reported that adding heart rate signal to a control-to-range AP system helped to protect T1DM patients against hypoglycemia during exercise.7 Ding and Schumacher have provided an excellent review of early work in using sensor monitoring of physical activity to improve glucose management.8
Physiologic sensors have been employed for other medical conditions, such as determination of heart rate and cardiac arrhythmias with skin electrodes, blood pressure with cuff-based monitors on the arm or finger, skin temperature with skin patch thermometers, oxygen saturation with pulse oximeters, respiratory rate by piezoelectric sensors, perspiration (sweating) with galvanic skin response (GSR) detectors, seizures via electroencephalograms, and body motion or activity with accelerometers. Commercially available fitness trackers and smart watches, which incorporate multiple physiologic sensors, are now becoming attractive devices to use for diabetes management. These trackers are relatively small, inexpensive, unobtrusive, noninvasive, and encourage a healthy lifestyle. They have also been enthusiastically embraced by large numbers of people both with and without diabetes. The signals from these sensors could provide information about the wearer’s environment and activities with minimal effort on the part of the wearer. For example, increased heart rate and/or GSR could indicate exercise without the need for the wearer to explicitly announce that activity. Exercise could then be more readily incorporated into algorithms to model blood glucose behavior, such as AP control algorithms.
Our previous work using artificial intelligence techniques to detect abnormal patterns in CGM/insulin pump data demonstrated that incorporating patient life-event data enhanced our ability to detect and determine the probable causes of abnormal glucose excursions two-fold.9 Therefore, we were curious to see if data from physiological sensors could enhance our ability to determine cause and effect or predict future glucose levels. In our early experimentation using fitness trackers to capture these data and combine them with CGM, insulin pump, and life event data, we have encountered reasons for optimism but also problems and frustrations. Here, we describe our own experience briefly, and then we detail the problems that will need to be overcome if wearable physiological sensors are to live up to their promise.
Machine Learning Terms and Approaches
In our experimentation, we employ machine learning approaches, including support vector machines (SVMs) and recurrent neural networks (RNNs) with long short-term memory (LSTM) units. SVMs and RNNs with LSTM units are approaches with broad applicability to a wide range of problems. Here, we briefly define these terms and provide references for the interested reader. The SVM is a machine learning algorithm that learns a decision boundary maximizing the margin between positive and negative examples. SVMs can project the training and test examples into an implicit high dimensional feature space, where dimensions correspond to combinations of original features, and efficiently learn linear separators in this implicit space.10,11 The RNN is a type of artificial neural network that contains units with recurrent connections. A neuron has a recurrent connection if its output at time step t is used as input at the next time step t + 1. Units with recurrent connections can function as memory, which theoretically enables RNNs to process and learn from arbitrary sequences and time series data.12 LSTM is a type of artificial neuron originally developed by Hochreiter and Schmidhuber13 to alleviate the vanishing gradient problem that prevents vanilla RNNs from learning long-range dependences in input sequences.14 A typical LSTM unit contains an input gate, an output gate, and a forget gate that control the functioning of an internal cell, which can theoretically remember values over arbitrarily long intervals.
Observations and Preliminary Work
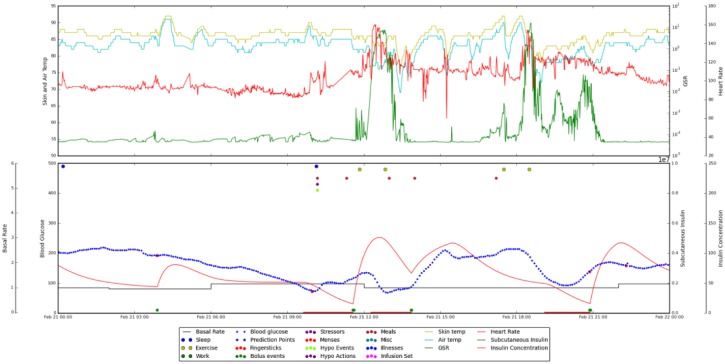
In a preliminary N-of-1 study, a patient with T1DM on insulin pump therapy with CGM wore a Basis Peak fitness band (Intel, Santa Clara, CA) and manually entered life-event data (meals, exercise, sleep) via cell phone for two months.15 We analyzed the resulting data, both statistically and visually. Using an in-house graphing package that allowed us to integrate and visualize these data, we were able to see some marked patterns. For example, when the patient shoveled snow, which is strenuous exercise, we could see increased GSR and heart rate with decreased skin and air temperature. This pattern can be seen in Figure 1. As shoveling snow precipitated hypoglycemia in this patient, we began investigating whether sensor signal patterns could help us to detect and/or predict hypoglycemia.
Figure 1.
Integrated data for a 24-hour period, from midnight to midnight. Data in the upper pane are from the Basis Peak band. Displayed are skin temperature (yellow), air temperature (cyan), heart rate (red), and GSR (green). The lower pane shows CGM data (blue), estimated insulin on board (red), and life events (colored dots at the top of the pane). The shoveling incidents occur at around noon and 6:00 pm. They are marked by drops in blood glucose level, increased GSR and heart rate, and decreased skin and air temperature.
In a subsequent study, we analyzed data from our original subject plus one additional subject. The additional subject was also a T1DM patient on insulin pump therapy, who supplied insulin, CGM, Basis Peak, and life-event data for two months. These subjects, one male and one female, were both middle-aged and had had T1DM since childhood. Empirical evaluations showed statistically significant correlations between blood glucose levels and a number of features derived from physiological parameters such as GSR, heart rate, skin and air temperature, sleep quality, and step counts. For example, nocturnal hypoglycemic episodes were observed to be statistically correlated with the variance in GSR measured over the previous 24 hours. The standard deviation of the difference between skin temperature and air temperature in the previous hour was a useful signal for detecting hypoglycemia that occurred during late evening hours.16 When trained on these and other manually engineered features to detect hypoglycemic episodes, linear and quadratic SVM models were shown to significantly outperform both a random guessing model and a thresholded GSR model.16 Although the sensor measurements from the Basis Peak band were not sufficient on their own for reliably detecting hypoglycemia, the results showed that they contained signals that are correlated with blood glucose behavior. This motivated us to switch from detection of hypoglycemic events to prediction of blood glucose levels using models that can automatically leverage any number of raw physiological parameters. In our recent work,17 we showed that RNNs with LSTM units can learn physiological models of blood glucose that are competitive with a previous state-of-the-art model based on manually engineered physiological equations, when using blood glucose history, insulin, and meal carbohydrate data. We are currently using this flexible LSTM approach to determine if physiological parameters acquired from sensor bands are useful not only for detection, but also for prediction of blood glucose levels.
Difficulties in Leveraging Wearable Sensor Technology
While we are still working to opportunistically leverage wearable physiological sensor data, we are finding this to be more difficult than anticipated. In particular, shortly after we began a larger study, the Basis Peak fitness bands we were using were recalled due to an issue with overheating, which could cause burns on the wrist. Our subjects had to remove the Basis Peak bands at once for their safety, and we were in the difficult position of trying to find replacement bands so that the study could continue. We have not yet found a commercially available wearable device that meets all of our research needs.
We purchased and evaluated three other commercially available fitness bands. We considered only bands with access to raw sensor data, which ruled out the popular Fitbit (Fitbit, San Francisco, CA) and Jawbone UP3 (Jawbone, San Francisco, CA). We spent considerable effort trying to integrate data from the Microsoft Band 2 (Microsoft, Redmond, WA) before it was pulled from the market. The Microsoft Band 2 had an application programming interface (API) that allowed collection of some raw data in real time. However, the data collection was sporadic. No data were collected when the user’s smartphone was out of the band’s signal range. As the band was wearable, but the smartphone could be set down and left behind, there were gaps in our evaluation data. The Angel band (Angel, Tel Aviv, Israel), developed by a startup company with Indiegogo funding, initially seemed very promising, as it offered multiple sensors in a small package with full access to raw sensor data. However, the company stopped selling or supporting the product before we could use the bands with patients. We are currently using Empatica’s Embrace band (Empatica Inc, Cambridge, MA), which was originally developed to detect seizures in patients with epilepsy.18 It includes accelerometer data, which were not available in the Basis band, but it does not include heart rate data. It is smaller and more comfortable to wear than the other bands we evaluated. A drawback is that its battery must be charged for two hours every day, which leaves gaps in the collected data. Furthermore, data are not yet available to technology developers in real time, but must be downloaded retrospectively. The company is currently working on a research portal to facilitate data downloads.
From a technology development perspective, desiderata are accurate measurements of physiological parameters, full access to raw data in real time, and all physiological sensors on the same platform. Raw data signals, not proprietary apps or plots, are needed for algorithm development. Unfortunately, few sensor bands make raw data available. We hypothesize that incentives for manufacturers may disincline them to provide raw data access. Proprietary apps may help manufacturers retain customers; the majority of customers, who are not technology developers, may not care about raw data access. Fitbit was sued over the inaccuracy of its heart rate data;19 it could be safer for manufacturers just not to provide it. It is also important for the raw data to be available in real time, via an API. While acquiring data retrospectively is useful for proof-of-concept research, real-time data are needed to generate alerts and reminders in time to be of benefit to patients. An additional complication for technology development is the need to use multiple devices, each with its own data format and data access protocol, to acquire different physiological signals. A complete sensor suite on one wristband or other wearable platform would facilitate technology development.
Potential difficulties for patients using the new technology are considered next. Patients with type 1 diabetes who use insulin pumps are already utilizing wearable technology. We now propose to add more devices, like fitness trackers, smart watches, sensor-embedded adhesive bandages, and even sensor-embedded clothing, to improve our ability to monitor physiological parameters and life events contributing to abnormal glucose excursions. However, this entails attaching more apparatus to the patient, adding to the daily burden of self-care, as effort is required to wear, charge, and otherwise attend to these additional devices. To minimize this burden, it will be necessary to seamlessly capture information from wearable physiological sensors, track it, and determine what is actionable for the patient and/or their health care team. Simple devices, which can automate data collection and analysis with minimal effort on the part of patients, are to be desired.
There are at least three further concerns, as the new technology is introduced to patients. First, it is important that using the new devices does not compromise the confidentiality of patients’ personal health information. The privacy and security of patient data must be assured. Second, the cost of additional devices must be reasonable, so as not to contribute to burgeoning health care costs. Finally, there should be some standardization of this equipment, allowing seamless integration of devices from different manufacturers, so that patients could pick the best devices for their own personal lifestyles.
While all authors of this commentary are technology developers, only the first author (FLS) is a physician. Here, he shares his own perspective on how wearable sensor technology could potentially impact physicians. First of all, physicians are already overloaded with data. They face demands to maintain electronic medical records documenting every patient interaction, whether face-to-face or remote, every laboratory and diagnostic test ordered or returned with results, and every patient communication via phone call or email. Physicians treating persons with diabetes are now receiving individual patient glucose records between visits, at daily, weekly, or other regular intervals. They must document their interpretation of this data as well as any therapeutic actions recommended. New data from wearable physiological sensors could potentially add to this data overload. If physicians are to use these new data to help them determine the impact of an activity, such as exercise, on glucose control and to recommend adjustments in diabetes management, the data must be automatically analyzed and interpreted and graphically presented in a clear and concise manner. Then, physicians would be able to concur or disagree with the automated data interpretation without incurring further data overload. Second, the data from wearables must provide unique and additional information which helps solve specific clinical problems in glucose control. For example, in our preliminary data reviews, we have observed increased GSR before the glucose sensor detects hypoglycemia, which, if confirmed, could provide an additional level of safety for preventing nocturnal hypoglycemia.
Table 1 provides a summary of the challenges identified for leveraging wearable physiologic sensors for diabetes management.
Table 1.
Challenges for Leveraging Wearable Physiologic Sensors for Diabetes Management.
| Accurate measurements of physiological parameters Full access to raw data in real time All needed sensors on a single wearable platform Minimal burden for patients Comfortable, unobtrusive devices Confidentiality of patients’ personal health information Data analysis without data overload for clinicians Standardization and integration with other medical devices Battery life Cost-effectiveness Market stability and availability of commercial devices |
Conclusions
With the technology still in its infancy, additional questions and issues still need to be considered. Will increasing technological complexity bring additional problems, such as those of connectivity? How will we monitor and manage potential equipment failures? Will the technology benefit some groups, like competitive athletes or those with hypoglycemia unawareness, more than others? What policy and regulatory issues will arise? Further study is needed to shed light on these questions, issues, and challenges. As the technology progresses, longer-term clinical studies will be needed to evaluate efficacy, clinical outcomes, and patient acceptance.
Wearable physiologic sensors could potentially aid in diabetes management by providing data that may (1) contribute to improved pump controller algorithms; (2) compensate for the limitations of sense-and-respond insulin pumps; (3) enhance patient safety, especially with regard to nocturnal hypoglycemia; (4) illuminate how patient activities impact blood glucose levels; (5) help determine cause and effect for blood glucose excursions; and (6) enable more accurate blood glucose level predictions. For this potential to be realized, it will be necessary to overcome the current difficulties in leveraging this technology and to ensure that the needs of patients and physicians are met. Efforts made now, while the technology is still young, could lead to substantially better ways of monitoring and managing diabetes as well as other chronic diseases.
Footnotes
Abbreviations: AP, artificial pancreas; API, application programming interface; CGM, continuous glucose monitoring; GSR, galvanic skin response; LSTM, long short-term memory; RNN, recurrent neural network; SVM, support vector machine; T1DM, type 1 diabetes mellitus.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: FLS is director of and has stock ownership in Intelligence 4 Diabetes Care.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This material is based upon work supported by the National Science Foundation under Grant Number 1117489 and by the National Institutes of Health under Grant Number 1R21EB022356.
References
- 1. Pettus J, Edelman SV. Recommendations for using real-time continuous glucose monitoring (rtCGM) data for insulin adjustments in type 1 diabetes. J Diabetes Sci Technol. 2017;11(1):138-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bally L, Thabit H, Tauschmann M, et al. Assessing the effectiveness of a 3-month day-and-night home closed-loop control combined with pump suspend feature compared with sensor-augmented pump therapy in youths and adults with suboptimally controlled type 1 diabetes: a randomised parallel study protocol. BMJ Open. 2017;7(7):e016738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Basu A, Dube S, Veettil S, et al. Time lag of glucose from intravascular to interstitial compartment in type 1 diabetes. J Diabetes Sci Technol. 2015;9(1):63-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Joslin Diabetes Center. Insulin action. Available at: http://www.joslin.org/info/insulin_action.html. Accessed January 26, 2018.
- 5. Stenerson M, Cameron F, Payne SR, et al. The impact of accelerometer use in exercise-associated hypoglycemia prevention in type 1 diabetes. J Diabetes Sci Technol. 2014;9(1):80-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schechter A, Eyal O, Zuckerman-Levin N, Amihai-Ben-Yaacov V, Weintrob N, Shehadeh N. A prototype of a new noninvasive device to detect nocturnal hypoglycemia in adolescents with type 1 diabetes—a pilot study. Diabetes Technol Ther. 2012;14(8):683-689. [DOI] [PubMed] [Google Scholar]
- 7. Breton MD, Brown SA, Karvetski CH, et al. Adding heart rate signal to a control-to-range artificial pancreas system improves the protection against hypoglycemia during exercise in type 1 diabetes. Diabetes Technol Ther. 2014;16(8):506-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ding S, Schumacher M. Sensor monitoring of physical activity to improve glucose management in diabetic patients: a review. Sensors (Basel). 2016;16(4):E589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schwartz FL, Vernier SJ, Shubrook JH, Marling CR. Evaluating the automated blood glucose pattern detection and case-retrieval modules of the 4 Diabetes Support System. J Diabetes Sci Technol. 2010;4(6):1563-1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vapnik V. Statistical Learning Theory. New York, NY: Wiley; 1998. [Google Scholar]
- 11. Schölkopf B, Smola AJ. Learning with Kernels: Support Vector Machines, Regularization, Optimization, and Beyond. Cambridge, MA: MIT Press; 2002. [Google Scholar]
- 12. Goodfellow I, Bengio Y, Courville A, Bengio Y. Deep Learning. Cambridge, MA: MIT Press; 2016. [Google Scholar]
- 13. Hochreiter S, Schmidhuber J. Long short-term memory. Neural Computation. 1997;9(8):1735-1780. [DOI] [PubMed] [Google Scholar]
- 14. Hochreiter S, Bengio Y, Frasconi P, Schmidhuber J. Gradient flow in recurrent nets: the difficulty of learning long-term dependencies. In: Kremer SC, Kolen JF, eds. A Field Guide to Dynamical Recurrent Neural Networks. New York, NY: IEEE Press; 2001:237-244. [Google Scholar]
- 15. Marling C, Bunescu RC, Baradar-Bokaie B, Schwartz F. Case-based reasoning as a prelude to big data analysis: a case study. In: Workshop Proceedings of the Twenty-Third International Conference on Case-Based Reasoning Frankfurt, Germany: CEUR; 2015:175-183. [Google Scholar]
- 16. Marling C, Xia L, Bunescu R, Schwartz F. Machine learning experiments with noninvasive sensors for hypoglycemia detection. In: IJCAI 2016 Workshop on Knowledge Discovery in Healthcare Data New York, NY: IJCAI; 2016:1-6. [Google Scholar]
- 17. Mirshekarian S, Bunescu R, Marling C, Schwartz F. Using LSTMs to learn physiological models of blood glucose behavior. In: 39th International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC’17) Jeju Island, Korea: IEEE; 2017:2887-2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Empatica. Embrace. Available at: https://www.empatica.com/product-embrace. Accessed January 26, 2018.
- 19. Fox News. Fitbit being sued for faulty heart rate tracking. Available at: http://www.foxnews.com/health/2016/01/07/fitbit-being-sued-for-faulty-heart-rate-tracking.html. Accessed January 26, 2018.