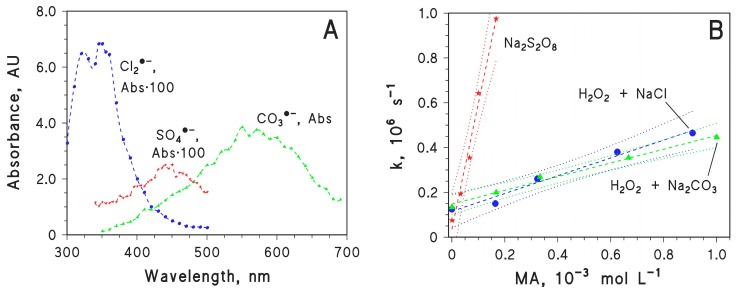
Figure 4.
(A) Absorption spectra of the radicals CO3·−, Cl2·−, and SO4·− produced by laser flash photolysis of 2.5 mM H2O2 + 0.1 M Na2CO3 (CO3·−), of 2.5 mM H2O2 + 0.01 M NaCl (pH 3, adjusted with HClO4) (Cl2·−), and of 10 mM Na2S2O8 (SO4·−). The absorbance signals were taken soon after the laser pulse. Laser irradiation at 266 nm, 35 mJ·pulse−1; (B) First-order decay constants of the studied radical species (SO4·−, Cl2·−, CO3·−) as a function of MA concentration. The radical species were obtained by laser irradiation of 10 mM Na2S2O8 (SO4·−), of 2.5 mM H2O2 + 0.01 M NaCl at pH 3 (Cl2·−), and of 2.5 mM H2O2 + 0.1 M Na2CO3 (CO3·−). The slopes of the lines give the second-order reaction rate constants between the relevant radicals and MA (Stern-Volmer approach).