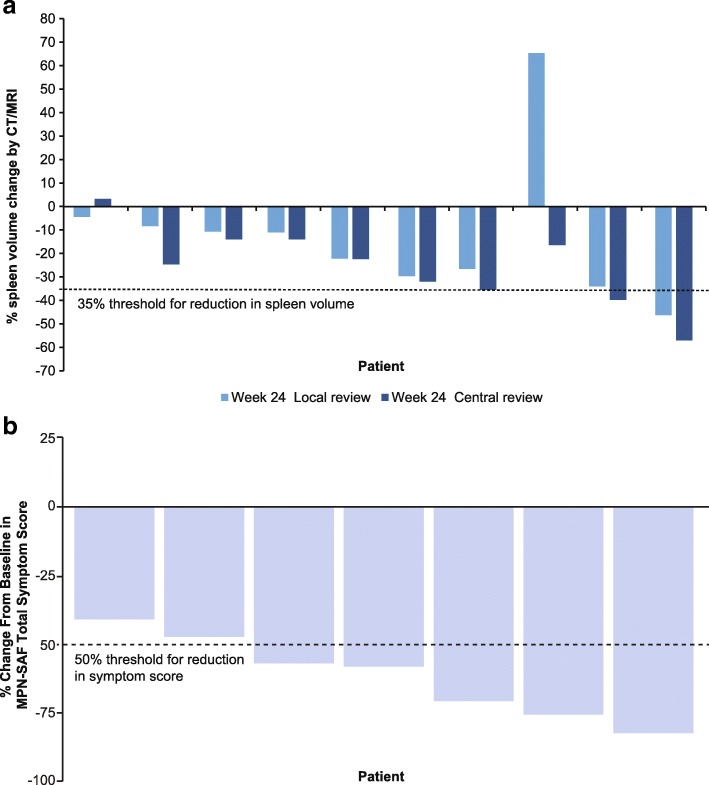
Fig. 1.
Spleen volume change from baseline per central and investigator review (a) and symptom response change (b) at week 24, by patient. Threshold for clinical efficacy at 35% reduction in spleen volume and 50% reduction in the MPN-SAF TSS is indicated by the dotted line. Spleen volume was assessed by CT or MRI, both by a local radiologist and a central independent review committee. Symptom score data were not available for three patients, including the patient who discontinued early. CT computed tomography; MPN-SAF TSS Myeloproliferative Neoplasm Symptom Assessment Form Total Symptom Score; MRI magnetic resonance imaging