Figure 8.
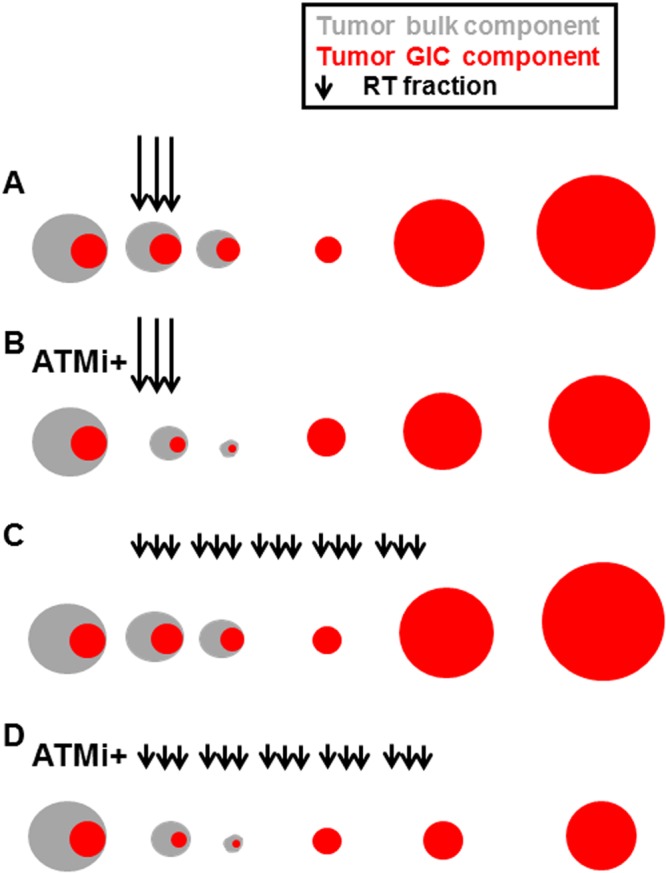
Proposed mechanism for in vivo radiosensitization by ATMi in GIC-driven orthotopic tumors. (A) RT initially exerts its limited cytocidal effect on the radioresistant, quiescent GIC component of glioma (indicated in red). Once RT is over, the GICs resume proliferation driving tumor progression. (B) ATMi such as KU60019 may radiosensitize the GICs and initially augment their killing by dislodging them from quiescence but, once RT is over, the persistent ATMi-mediated stimulus to proliferate causes the GIC recovery and expansion, thus mitigating the initial radiosensitization benefit. (C) Hyperfractionation of RT has little or no beneficial effect in limiting tumor progression as compared to standard RT fractionation in (A). (D) In the presence of ATMi, RT hyperfractionation may allow to take advantage of the long-term proliferation-boosting, radiosensitizing effect of the drug towards the GIC component of the tumor and significantly delay tumor progression.
