Fig. 6. Conformational changes upon GPCR activation.
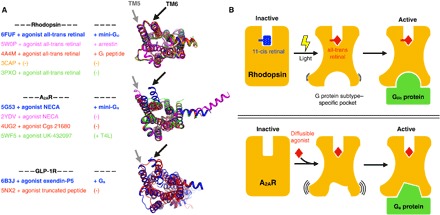
(A) Comparison between active conformations of rhodopsin, A2AR, and GLP-1R, respectively. We chose these receptors because their structures have been solved in different active and intermediate-active states. The superimposed structures are shown from the cytoplasmic side. We highlight the location of TM5 and TM6 to assist in visualizing the opening of the effector-binding site. (B) The top panel depicts the structural intermediates during rhodopsin activation. Light-induced retinal isomerization results in the formation of a predefined binding pocket for a Gi/o protein or arrestin. On the other hand, the bottom panel depicts how binding of diffusible ligands results in the formation of transient active GPCR conformations with higher conformational heterogeneity than active rhodopsin. In this case, the G protein likely selects and stabilizes a suitable conformation of the binding pocket from this ensemble.
