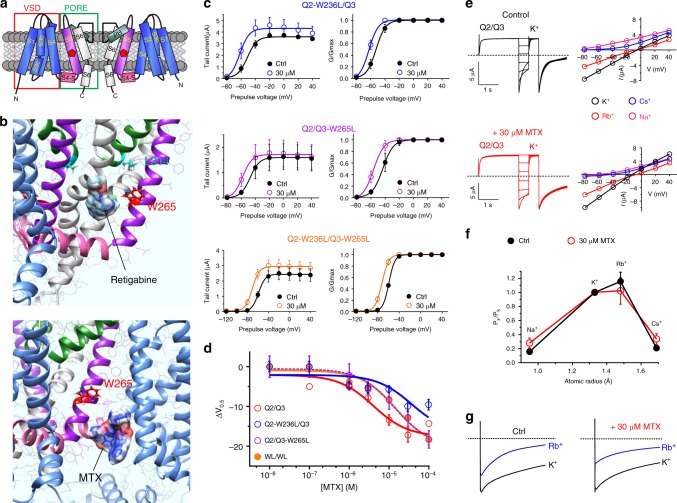
Fig. 3.
MTX activates KCNQ2/3 by binding close to the pore. a KCNQ topology (two of four subunits shown) indicating approximate position of KCNQ3-W265. VSD voltage-sensing domain. b Binding position of (upper) retigabine and (lower) MTX in KCNQ3 predicted by SwissDock using a chimeric KCNQ1–KCNQ3 structure model. c Effects of MTX (30 µM) on tail current and G/Gmax relationships for single- and double-W/L mutant KCNQ2/3 channels as indicated (n = 3–5). Voltage protocol as in Fig. 1d. d Dose response for mean ΔV0.5 of activation induced by MTX for wild-type KCNQ2/3 and mutant channels as in c (n = 3–9). e Left, exemplar traces; right, mean I/V relationships for KCNQ2/3 channels bathed in 100 mM K+, Rb+, Cs+, or Na+ in the presence or absence (Control) of MTX (30 µM); n = 4–7. f Relative ion permeabilities of KCNQ2/3 channels in the presence or absence (Ctrl) of MTX (30 µM); n = 4–7. Quantified from traces and plots as in panel e. g Relative Rb+ to K+ permeabilities of KCNQ2/3 channels in the presence or absence (Ctrl) of MTX (30 µM); n = 4–8. All error bars indicate SEM