Fig. 2. Ion conductance in response to nucleotide binding.
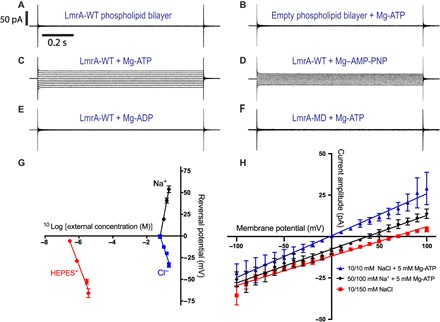
(A to F) Macroscopic current responses to the imposition of the same voltage protocol as in Fig. 1, in symmetric 10 mM HEPES, 20 mM EGTA, and 10 mM NaCl solutions (pH 7.2, NaOH) in the absence or presence of 5 mM Mg-nucleotide in the external buffer. Scale bars in (A) are applicable to all current responses. (G) Determination of ion stoichiometry from Erev measurements. Erev was measured in the presence of 5 mM Mg-ATP as a function of the log10 of the external concentration of single buffer components: Na+ (black circle), Cl− (blue square), or monovalent cationic HEPES+ (red diamond). Data points (±SEM) show the mean Erev for at least three independent measurements. The internal concentration of Na+ and Cl− was maintained at 60 mM, and the external concentration was raised from 60 to 100, 150, or 200 mM, with HEPES maintained at 10 mM in internal and external solutions. To measure the effect of HEPES (pH 6.5), the internal solution contained 60 mM NaCl, 20 mM EGTA, and 10 mM HEPES. The external HEPES concentration was raised from 10 to 25, 100, or 125 mM, yielding HEPES+ concentrations of 0.29, 0.73, 2.9, and 3.6 μM. The ion stoichiometry was determined from the slope of the linear fit using the Nernst equilibrium equation (data analysis S1). The slope values for Na+, Cl−, and HEPES+ were 104.6 ± 5.3 mV, −59.1 ± 7.8 mV, and −51.1 ± 4.9 mV, respectively (n = 3). (H) Current-voltage relation (I-V) response graph for LmrA-WT using symmetric solutions as listed under (A) with 5 mM Mg-ATP in the external buffer (blue trace), or asymmetric solutions containing [Na+]in/[Na+]out = 50 mM/100 mM and [Cl−]in/[Cl−]out = 50 mM/50 mM in the presence of the ATP (black trace), or containing [NaCl]in/[NaCl]out = 10 mM/150 mM without nucleotide (red trace). The Erev for the blue, black, and red traces was −2.0 ± 1.2 mV, 37.6 ± 1.5 mV, and 66.7 ± 6.1 mV (n = 3), respectively.
