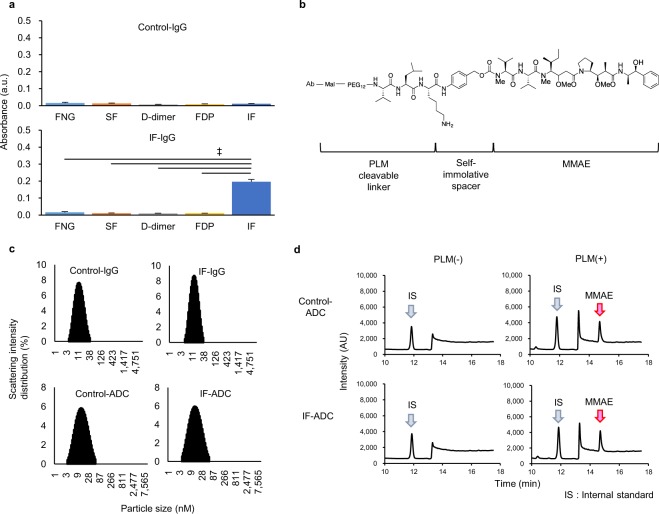
Figure 1.
Characterization of anti-IF mAb and IF-ADC. (a) Specificity of anti-IF IgG. Reactivities of control antibody and anti-IF antibody were estimated against fibrinogen (FNG), soluble fibrin (SF), D-dimer, fibrin degradation product (FDP) and IF by ELISA (n = 3). Anti-IF mAb specifically recognised IF, but did not recognised its zymogen FNG and its degradation products FDP and D-dimer, whereas control mAb did not recognised FNG, IF, FDP and D-dimer. The results are shown as means ± standard deviation (s.d.). Statistical analysis was performed using the Dunnett test. ‡P < 0.01. (b) Structure of antibody-drug conjugate. MMAE was conjugated to mAb via PLM cleavable linker and self-immolative spacer. (c) Particle size of mAbs and ADCs. The molecular sizes of the ADCs did not change as compared to that of the corresponding antibody. This may implicate that ADCs were not fragmented or aggregated. (d) Chromatography of released MMAE. The peak at a retention time of 11.8 min is the ibuprofen (IS) peak (blue arrow), and the peak at a retention time of 14.7 min indicates MMAE (red arrow). Control- and IF-ADC released MMAE only when PLM was added, but there was virtually no MMAE release in the absence of PLM.