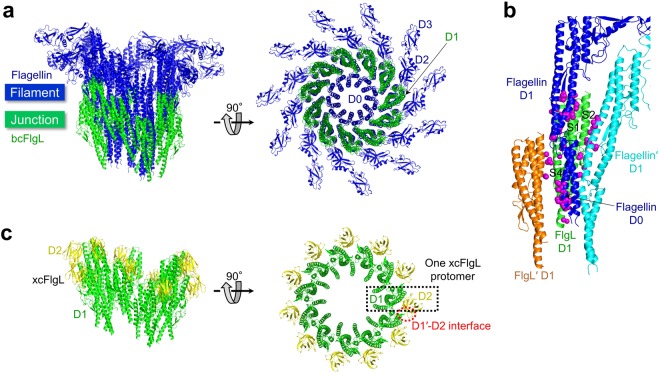
Figure 6.
Structural model of FlgL and flagellin assembly in the flagellum. (a) FlgL-flagellin model. To build a model that consists of 11 FlgL protomers (green) and 11 flagellin protomers (blue), 22 flagellin protomers that constitute two continuous layers were obtained from the cryo-EM structure of the S. Typhimurium filament, and the bottom 11-repeat flagellin layer was overlaid and replaced with 11 bcFlgL D1 domains. S. Typhimurium flagellin consists of four domains, D0–D3. The conserved D0 and D1 domains of S. Typhimurium flagellin occupy the inner and middle rings of the filament, respectively, and are responsible for inter-flagellin interactions. However, the hypervariable D2 and D3 domains radiate to the outside and do not mediate flagellin polymerization. The structure of the bcFlgL D1 domain is superimposable on that of the flagellin D1 domain and assembles into the middle ring in the flagellum. (b) Interactions of FlgL (green ribbons) with its adjacent FlgL molecule, FlgL′ (orange ribbons), and flagellin molecules (blue and cyan ribbons) in the FlgL-flagellin assembly model shown in Fig. 6a. FlgL′, flagellin, and flagellin′ residues that make contact with FlgL are represented by magenta spheres. The S1, S2, and S4 segments of FlgL, which interact with adjacent molecules, are labeled. (c) Structural model of the xcFlgL D1 (green) and D2 (yellow) domains in the flagellum. One xcFlgL protomer is boxed in a black dotted rectangle. The binding interface between FlgL D2 and FlgL′ D1 is highlighted with a red dotted circle.