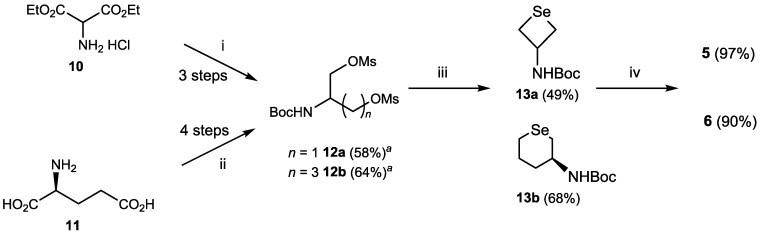
Scheme 2.
Synthesis of monoamino selenides 5 and 6. Reagents and conditions: (i) (1) Boc2O, Et3N, 1,4-dioxane/H2O (5:2), 50 °C, 18 h, (2) NaBH4, EtOH, reflux, 1 h, (3) MsCl, Et3N, CH2Cl2, 20 h, room temperature (rt); (ii) (1) EtOH, AcCl, reflux, 4 h, (2) Boc2O, Et3N, 1,4-dioxane:H2O = 5:2, 50 °C, 18 h, (3) NaBH4, EtOH, reflux, 1 h, (4) MsCl, Et3N, CH2Cl2, 20 h, rt; (iii) NaHSe, iPrOH/1,4-dioxane, reflux, 2.5 h for synthesis of 13a from 12a; NaHSe, EtOH/THF, reflux, 3 h for synthesis of 13b from 12b; (iv) HCl, H2O/EtOH, 35 °C, 20 h. a Details of the synthesis are given in Supporting Information.