Figure 4.
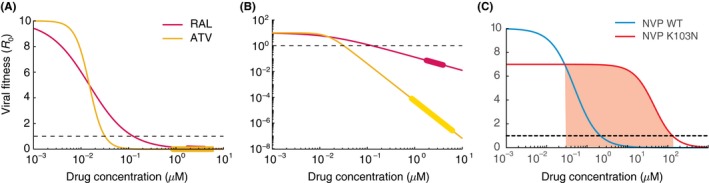
Effects of antiretroviral drugs on viral infectivity. (A) Dose‐response curves measured in single‐round ex vivo assays for the integrase inhibitor raltegravir (RAL, IC 50 = .015 uM, m = 1) and the protease inhibitor atazanavir (ATV, IC 50 = .015 uM, m = 2.9). The assay measures f u (Equation [Link]), the fraction of uninhibited infections compared to the absence of drug, and we scale this up to effective basic reproductive number by multiplying by drug‐free R 0 = 10. Below R 0 = 1 infection be controlled. (B) Same as A but plotted on log‐log scale to highlight differences in suppression between drugs at clinically relevant concentrations. The thicker regions on the lines are drug levels between the typical peak and trough concentrations when drug is taken daily with perfect adherence. (C) Dose‐response curves for wildtype (blue) and the K103N mutant (red) for the NNRTI nevirapine (NVP). Red shaded area is the “mutant selection window”, the range of drug concentrations where a resistant strain could outcompete wildtype and cause treatment failure. Parameters taken from.67, 69
