Table 2.
Heck reaction of aryl halides and n-butyl acrylate catalyzed by OCMCS-Ni a.
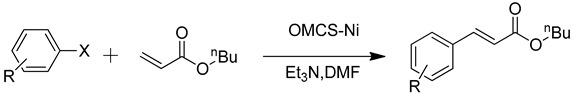
| Entry | ArX | Product | Yield b/% |
|---|---|---|---|
| 1 | 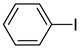 |
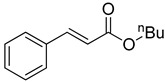 |
94 |
| 2 | 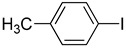 |
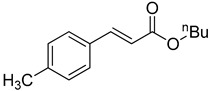 |
86 |
| 3 | 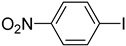 |
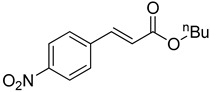 |
88 |
| 4 | 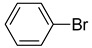 |
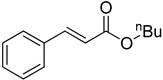 |
67 |
| 5 | 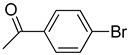 |
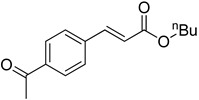 |
79 |
| 6 | 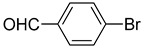 |
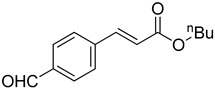 |
72 |
| 7 | 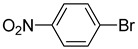 |
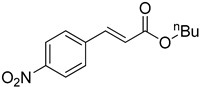 |
87 |
| 8 | 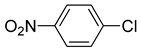 |
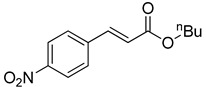 |
- |
| 9 | 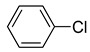 |
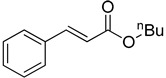 |
- |
a Reaction conditions: aryl halides (1.0 mmol), n-butyl acrylate (1.1 mmol); OCMCS-Ni (0.04 mmol Ni), DMF (5.0 mL) at 140 °C for 12.0 h; b The reaction yields were determined by GC-MS analysis of the crude reaction product.
