Table 1.
Structures of TBZ derivatives.
| No. | R1 | R2 | R3 | R4 | R5 | R6 |
|---|---|---|---|---|---|---|
| TBZ | H | H | H | H | H | 4-thiazole |
| TBZ-01 | H | Me | H | H | H | 4-thiazole |
| TBZ-02 | Me | H | H | H | H | 4-thiazole |
| TBZ-03 | H | Me | Me | H | H | 4-thiazole |
| TBZ-04 | H | H | Cl | H | H | 4-thiazole |
| TBZ-05 | H | H | F | H | H | 4-thiazole |
| TBZ-06 a | H | H | OMe | H | H | 4-thiazole |
| TBZ-07 | H | Bz | H | H | H | 4-thiazole |
| TBZ-08 | H |  |
H | H | 4-thiazole | |
| TBZ-09 | H | H | NO2 | H | H | 4-thiazole |
| TBZ-10 | H | H | H | H | Me | 4-thiazole |
| TBZ-11 | H | H | H | H | Bn b | 4-thiazole |
| TBZ-12 | H | H | H | H | pMB b | 4-thiazole |
| TBZ-13 | H | H | H | H | mNB b | 4-thiazole |
| TBZ-14 | Me | H | H | H | H | 2-Py b |
| TBZ-15 | H | 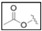 |
H | H | H | 4-thiazole |
| TBZ-16 | H | H | H | 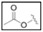 |
H | 4-thiazole |
| TBZ-17 | H | H | CF3 | H | H | 4-thiazole |
| TBZ-18 | H | H | H | H | 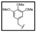 |
4-thiazole |
| TBZ-19 | H | H | H | H | Bn b | Bn b |
| TBZ-20 | H | H | H | H | 2-Py b | 2-Py b |
a Tautomer was determined by NMR spectroscopy analysis; b Bn= benzyl; pMB = p-methoxyl benzyl; m-NB = m-nitro benzyl; 2-Py = 2-pyridyl; Bz = benzoyl.
