Table 2.
Microbial producers of AChEIs.
| Strain | Classification | Structure of Active Ingredient(s) | Compound Type | IC50 | Method Used | Ref. |
|---|---|---|---|---|---|---|
| Aspergillus terreus |
Eumycota, Deuteromycotina, Hyphomycetes, Moniliales, Moniliaceae, Aspergillus |
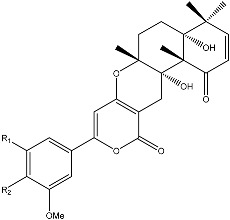 |
Territrem B | 7.6 μM | Ellman’s method | Ling et al. [25] |
|
Aspergillus terreus
(No. GX7-3B) |
Eumycota, Deuteromycotina, Hyphomycetes, Moniliales, Moniliaceae, Aspergillus |
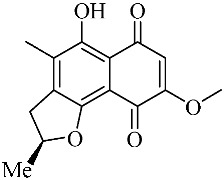 |
Anhydrojavanicin | 2.01 μM | Modified Ellman’s method | Deng et al. [26] |
 |
8-O-Methylbostrycoidin | 6.71 μM | ||||
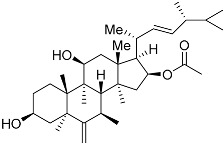 |
NGA0187 (Terpenoid) | 1.89 μM | ||||
 |
Beauvericin | 3.09 μM | ||||
| Aspergillus flavus LF40 |
Eumycota, Deuteromycotina, Hyphomycetes, Moniliales, Moniliaceae, Aspergillus |
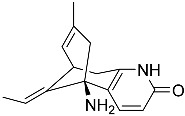 |
Huperzine A | 0.6 μM | Modified Ellman’s method | Wang et al. [27] |
| Aspergillus flavus |
Eumycota, Deuteromycotina, Hyphomycetes, Moniliales, Moniliaceae, Aspergillus |
 |
(8E,12Z)-10,11-Dihydroxyoctadeca-8,12-dienoic acid | No report | Modified Ellman’s method | Qiao et al. [28] |
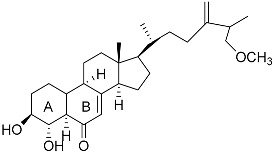 |
3β,4α-Dihydroxy-26-methoxyergosta-7,24(28)-dien-6-one | |||||
|
Aspergillus versicolor
Y10 |
Eumycota, Deuteromycotina, Hyphomycetes, Moniliales, Moniliaceae, Aspergillus |
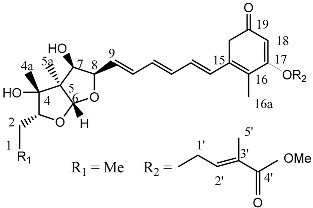 |
Avertoxin B | 14.9 μM | Modified Ellman’s method | Wang et al. [29] |
| Penicillium citrinum |
Eumycota, Deuteromycotina, Hyphomycetes, Moniliales, Moniliaceae, Penicillium |
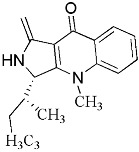 |
Quinolactacins A1 | 280 μM | Modified Ellman’s method | Kim et al. [30] |
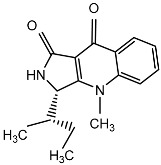 |
Quinolactacins A2 | 19.8 μM | ||||
|
Penicillium sp. EPF-6 |
Eumycota, Deuteromycotina, Hyphomycetes, Moniliales, Moniliaceae, Penicillium |
 |
Quinolactacins A, B, and C | No report | Ellman’s method | Kakinuma et al. [31] |
|
Penicillium sp. FO-4259 |
Eumycota, Deuteromycotina, Hyphomycetes, Moniliales, Moniliaceae, Penicillium |
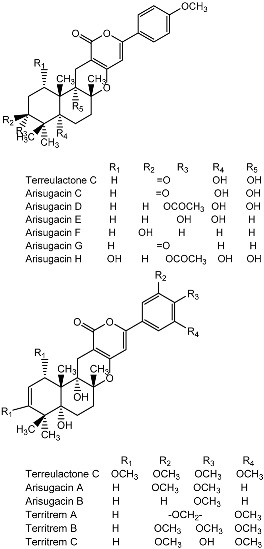 |
Arigsugacin I | 0.64 μM | Modified Ellman’s method | Omura et al.; Huang et al. [32,33] |
|
Penicillium sp. sk5GW1L |
Eumycota, Deuteromycotina, Hyphomycetes, Moniliales, Moniliaceae, Penicillium |
|||||
| Arigsugacins F | 0.37 μM | |||||
| Territrem B | 7.03 μM | |||||
|
Penicillium chermesinum
(ZH4-E2) |
Eumycota, Deuteromycotina, Hyphomycetes, Moniliales, Moniliaceae, Penicillium |
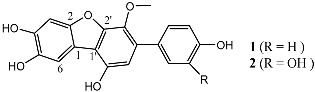 |
Terphenyls | 7.8 μM 5.2 μM |
Modified Ellman’s method | Huang et al. [34] |
|
Penicillium griseofulvum
LF146 |
Eumycota, Deuteromycotina, Hyphomycetes, Moniliales, Moniliaceae, Penicillium |
 |
Huperzine A | 0.6 μM | Modified Ellman’s method | Wang et al. [35] |
|
Paecilomyces tenuis
YS-13 |
Eumycota, Deuteromycotina, Hyphomycetes, Paecilomyces Paecilomyces |
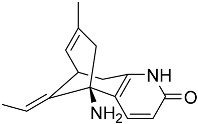 |
Huperzine A | 0.6 μM | Modified Ellman’s method | Su and Yang [36] |
|
Acremonium implicatum
LF30 |
Eumycota, Deuteromycotina, Hyphomycetes, Moniliales, Moniliaceae, Acremonium |
No clear products | Modified Ellman’s method | Wang et al. [35] | ||
|
Acremonium sp. 2F09P03B |
Eumycota, Deuteromycotina, Hyphomycetes, Moniliales, Moniliaceae, Acremonium |
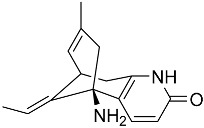 |
Huperzine A | 0.6 μM | No report | Li et al. [37] |
|
Botrytis sp. HA23 |
Eumycota, Deuteromycotina, Hyphomycetes, Moniliales, Moniliaceae, Botrytis |
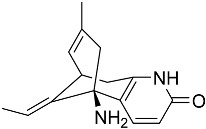 |
Huperzine A | 0.6 μM | No report | Ju et al. [38] |
| Trichoderma sp. |
Eumycota, Deuteromycotina, Hyphomycetes, Moniliales, Moniliaceae, Trichoderma |
No clear products | Modified Ellman’s method | Dong et al. [39] | ||
|
Colletotrichum gloeosporioides
ES026 |
Eumycota, Deuteromycotina, Coelomycetes, Melanconiales, Melanconiaceae Colletotrichum |
 |
Huperzine A | 0.6 μM | Modified Ellman’s method | Zhao et al. [40] |
|
Alternaria sp. YD-01 |
Eumycota, Deuteromycotina, Hyphomycetes, Moniliales, Dematiaceae Alternaria |
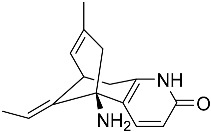 |
Huperzine A | 0.6 μM | TLC bioautography | Yang et al. [41] |
|
Cladosporium cladosporioides
LF70 |
Eumycota, Deuteromycotina, Hyphomycetes, Moniliales, Dermateaceae, Cladosporium |
 |
Huperzine A | 0.6 μM | ||
| Phomopsis sp. |
Eumycota, Deuteromycotina, Coelamycetes, Sphaeropsidales, Sphaeropsidaceae, Phomopsis |
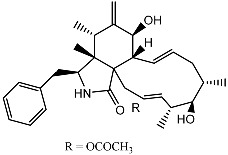 |
Cytochalasin H | No report | TLC bioautography | Chapla et al. [42] |
| Chrysosporium sp. |
Eumycota, Ascomycotina, Ascomycetes, Onygenales, Onygenaceae, Chrysosporium |
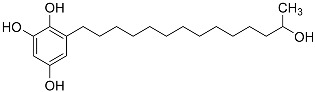 |
14-(2′,3′,5′-Trihydroxyphenyl)tetradecan-2-ol | 197 μM | Modified Ellman’s method | Sekhar Rao et al. [43] |
|
Shiraia sp. Slf14 |
Eumycota, Ascomycotina, Pyrenomycetes, Sphaeriales, Hypocreaceae, Shiraia |
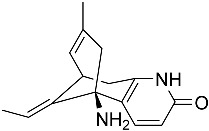 |
Huperzine A | 0.6 μM | Modified Ellman’s method | Zhu et al. [44] |
|
Xylaria sp. YS-02 |
Eumycota, Ascomycota, pyrenomycetes, Sphaeriales, Xylariaceae, Xylaria |
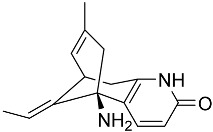 |
Huperzine A | 0.6 μM | Modified Ellman’s method | Su et al. [45] |
| Xylaria sp. |
Eumycota, Ascomycota, pyrenomycetes, Sphaeriales, Xylariaceae, Xylaria |
 |
Xyloketal A | 29.9 μM | Modified Ellman’s method | Lin et al. [46] |
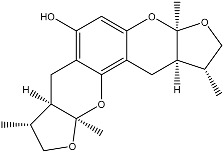 |
Xyloketal B | 109.3 μM | ||||
 |
Xyloketal C | 109.3 μM | ||||
 |
Xyloketal D | 425.6 μM | ||||
|
Hyalodendriella sp. Ponipodef12 |
Eumycota, Ascomycotina, Discomycetes, Leotiales, Leotiaceae, Hyalodendriella |
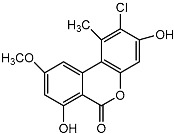 |
Palmariol B (1) | 115.31 µg/mL | Modified Ellman’s method | Meng et al. [47], Mao et al. [48] |
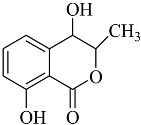 |
4-Hydroxymellein (2) | 116.05 µg/mL | ||||
 |
Alternariol 9-methyl ether (3) | 135.52 µg/mL | ||||
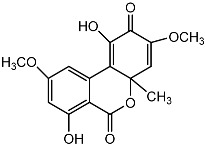 |
Botrallin (4) | 83.70 µg/mL | ||||
| Blastomyces sp. |
Eumycota, Ascomycotina, Hemiascomycetes, Endomycetalcs, Endomycetaccae, Blastomyces |
 |
Huperzine A | 0.6 μM | No report | Ju et al. [38] |
| Haddowia longipes |
Eumycota, Basidiomycotina, Hymenomycetes, Aphyllophorales, Ganodermataceae, Haddowia |
 |
11-Oxo-ganoderiol D (1) Lanosta-8-en-7,11-dioxo-3b-acetyloxy-24,25,26-trihydroxy (2) Lanosta-8-en-7-oxo-3b-acetyloxy-11b,24,25,26-tetrahydroxy (3) Ganoderiol D (10) Lanosta-8-en-7-one-3b-acetyloxy-24,25,26-trihydroxy (11) |
No report | Modified Ellman’s method | Zhang et al. [49] |
 |
Lanosta-7,9(11)-dien-3b-acetyloxy-24,25,26-trihydroxy (4) Ganodermanondiol (12) Lucidumol B (13) |
|||||
 |
11β-Hydroxy-lucidadiol (7) Lucidadiol (15) |
|||||
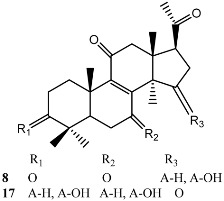 |
Lucidone H (8) lucidadone A (17) |
|||||
| Bacillus subtilis | Bacteria, Firmicutes, Bacilli, Bacillales, Bacillaceae, Bacillus |
No clear product | TLC bioautography and Modified Ellman’s method | Pandey et al.; Wang et al. [50,51] | ||
|
Streptomyces sp. AH-14 |
Phylum Actinobacteria, Actinomycetales, Streptomycetaceae, Streptomyces |
 |
Physostigmine | 41 μM | Modified Ellman’s method | Murao and Hayashi [52] |
|
Streptomyces sp. LB173 |
Phylum Actinobacteria, Actinomycetales, Streptomycetaceae, Streptomyces |
 |
Geranylphenazinediol | 2.62 μM | Modified Ellman’s method | Ohlendorf et al. [53] |
| Streptomyces lavendulae |
Phylum Actinobacteria, Actinomycetales, Streptomycetaceae, Streptomyces |
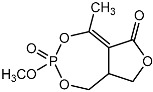 |
Oxygen heterocyclic compound | 7.6 μM | Modified Ellman’s method | Kurokawa et al. [54] |
| Rubrobacter radiotolerans |
Phylum Actinobacteria, Rubrobacteridae, Rubrobacterales, Rubrobacteraceae, Rubrobacter |
 |
2-(2-(3-Hydroxy-1-(1H-indol-3-yl)-2-methoxypropyl)-1H-indol-3-yl)acetic acid | 11.8 μM | Modified Ellman’s method | Li et al. [55] |
 |
3-(3-(2-Hydroxyethyl)-1H-indol-2-yl)-3-(1Hindol-3-yl)propane-1,2-diol | 13.5 μM | ||||
| N98-1021 |
Phylum Actinobacteria, Actinomycetales |
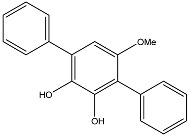 |
Same structure as terferol | 20 μM | Modified Ellman’s method | Dong et al. [56] |
| Streptosporangium sp. |
Phylum Actinobacteria, Actinomycetales, Streptomycetaceae, Streptosporangium |
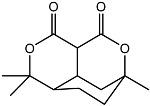 |
YXJ-E1 Novel compound extracted from secondary metabolites of Actinobacteria for the first time |
No report | Modified Ellman’s method | Yang et al. [57] |
 |
7,4′-Dihydroxy flavone | No report | ||||
| Talaromyces sp. LF458 |
Phylum Actinobacteria, Actinomycetales, Arthrobacter Talaromyces |
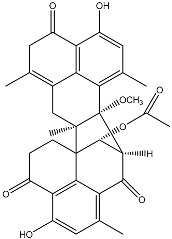 |
Talaromycesone A | 7.49 μM | Modified Ellman’s method | Wu et al. [58] |
| Cladonia macilenta Hoffm. | Lichenes, Ascolichens, Cladoniaceae, Cladonia |
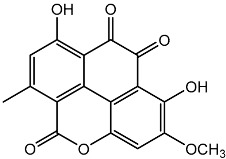 |
Biruloquinone 9,10-Phenanthrene quinone, a rare natural quinone compound |
27.1 μg/mL | Modified Ellman’s method | Luo et al. [59] |
| Nostoc 78-12A | Cyanophyta, Nostocales, Nostocaceae, Nostoc |
 |
Nostocarboline | 5.3 μM | Modified Ellman’s method | Becher et al. [60] |
