Abstract
Bone ongrowth on the surfaces of titanium (Ti)-coated polyetheretherketone (PEEK) materials has been demonstrated in animal models; however, whether this occurs on the surfaces of Ti-coated PEEK cages in lumbar interbody fusion has not been demonstrated clinically in vivo. This prospective observational study was aimed to develop and validate a computed tomography (CT) color mapping based on Hounsfield unit (HU) values for evaluation of bone ongrowth on the surfaces of the Ti-coated PEEK cage after posterior lumbar interbody fusion (PLIF).
Twenty-four consecutive patients (11 men and 13 women; mean age, 67.0 years; range, 20–82 years) who underwent single- or 2-level PLIF since March 2015 were included. Two Ti-coated PEEK cages were inserted in all PLIF segments. From reconstructed sagittal planes from postoperative CT scans (within 1 week and 6 months postoperatively), bone ongrowth on the surfaces of cage frames was evaluated by CT color mapping. Inter- and intraobserver reliability of the assessment of bone ongrowth by CT color mapping was evaluated by Cohen's kappa coefficient. The relation between CT color mapping and HU values on the surfaces of cage frames was also analyzed.
A total of 248 surfaces of cage frames were evaluated. Bone ongrowth was observed in 134 of 248 surfaces (54.0%) by CT color mapping. Intraobserver reliability for the evaluation of bone ongrowth was kappa = 0.831, and interobserver reliability was kappa = 0.713. The HU values in the local regions of interest (ROIs) on the surfaces of cage frames where the postoperative bone ongrowth existed on CT color mapping increased significantly postoperatively (P < .001), and the median postoperative change rate of the HU values in the local ROIs was 22.4%.
The assessment of bone ongrowth on the surfaces of Ti-coated PEEK cages by CT color mapping had adequate inter- and intraobserver reliability, which was useful especially in detecting local increase in HU values on the surfaces of the cages. This method is an easy and visually comprehensible method for the assessment of bone ongrowth in the bone-implant interface.
Keywords: bone ongrowth, color mapping, Computed tomography, intervertebral cage, polyetheretherketone, posterior lumbar interbody fusion, titanium-coated
1. Introduction
Various intervertebral cages made of different kinds of materials are available for lumbar interbody fusion surgery. Polyetheretherketone (PEEK) is 1 of the most widely used cage materials. The radiolucency of PEEK can help surgeons to evaluate fusion status.[1,2] The elastic modulus of PEEK, which is similar to that of cancellous bone, is favorable for reducing the risk of postoperative subsidence of PEEK cages.[2,3]
However, the lack of osseoconductivity and the hydrophobic nature of PEEK can have negative impacts on the primary stability of PEEK cages and the promotion of bony fusion.[4–7] Nemoto et al[8] showed that the fusion rate after transforaminal lumbar interbody fusion with the PEEK cages was inferior to that with titanium (Ti) cages and suggested that this difference might result from the chemical inertness of PEEK. As a solution to this concern about PEEK cages, Ti plasma spray coating for PEEK cages has been developed.
The main advantage of a Ti-coated PEEK cage is its bioactivity on the Ti-coated surfaces of the cage, with the elastic modulus of PEEK maintained. The progression of bone ongrowth or osseointegration on the surfaces of Ti-coated PEEK materials was demonstrated in animal models.[7,9] Furthermore, several authors reported favorable clinical outcomes after spinal arthrodesis surgery with Ti-coated PEEK cages.[10–12] However, whether bone ongrowth or osseointegration occurs on the surfaces of Ti-coated PEEK cages in lumbar interbody fusion has not been demonstrated clinically in vivo. One reason for this is that it is difficult to evaluate bone ongrowth or osseointegration by conventionally used clinical imaging modalities.
Recently, several authors have reported methods for evaluation of bone ongrowth on the surfaces of metal implants using clinical computed tomography (CT) scans.[13–16] The purpose of the present study was to establish and evaluate bone ongrowth on the surfaces of the Ti-coated PEEK cage after posterior lumbar interbody fusion (PLIF) by CT color mapping based on Hounsfield unit (HU) values.
2. Methods
Prospectively collected data approved by the research ethics committee of our institution were used in this study. The research protocol was approved and publicized by our institution, and patients were given the right to opt out of the study.
After exclusion of patients with scoliosis (Cobb angle > 10°), patients with rheumatoid arthritis, and those undergoing dialysis for chronic renal failure, 24 patients (11 men and 13 women) who underwent single- or 2-level PLIF (including those undergoing concomitant laminectomies at other levels) for the treatment of degenerative lumbar diseases (degenerative or isthmic spondylolisthesis, or foraminal stenosis) since March 2015 were included. Seventeen patients underwent single-level PLIF and 7 patients underwent 2-level PLIF. The mean (± standard deviation) age at the time of surgery was 67.0 ± 13.3 years (range, 20–82 years).
2.1. Surgical procedure
PLIF was performed by the conventional open method with bilateral total facetectomy, using Ti-coated PEEK cages (ProSpace XP, Aesculap AG, Tuttlingen, Germany) (Fig. 1) and pedicle screws and rods (CD HORIZON SOLERA System, Medtronic Sofamor Danek, Memphis, TN). After removal of the disc material and preparation of the vertebral endplates taking care not to break the endplates, 2 Ti-coated PEEK cages were inserted in each intervertebral space. Local autologous bone was used for bone graft material in the cages and intervertebral space in all cases, without bone morphogenic protein or allograft bone. Partial laminectomy was also performed in cases with concomitant canal stenosis at other levels.
Figure 1.

ProSpace XP cage (courtesy of B. Braun Aesculap Japan, Tokyo, Japan).
2.2. Demographic and clinical characteristics
Patient data, including age at the time of surgery, sex, and the level of PLIF segments, were obtained from medical charts and operative notes. In 19 patients, preoperative bone mineral density (T-score) at the lumbar spine (L2–L4) and hip (total) were measured by dual-energy X-ray absorptiometry (Discovery DXA System, Hologic, Marlborough, MA).
2.3. CT image acquisition and image analysis
CT images were acquired within 1 week and 6 months postoperatively on either of the 2 scanners (Discovery CT750 HD, GE Healthcare Japan, Tokyo, Japan; Aquilion ONE, Toshiba Medical Systems Corporation, Tochigi, Japan). The settings used for the scans were as follows: slice thickness, 0.625 mm with the Discovery CT750 HD and 0.5 mm with the Aquilion ONE; tube voltage, 120 kVp; matrix, 512 × 512; algorithm, standard. The tube current was maintained by an automatic exposure control system. Multiplanar reconstruction was performed in our institution by built-in 3-dimensional imaging software (Synapse Vincent; Fujifilm Holdings Corporation, Tokyo, Japan). A sagittal plane was adjusted to the plane containing the cage frame that formed the lateral wall of each cage (Fig. 2).
Figure 2.
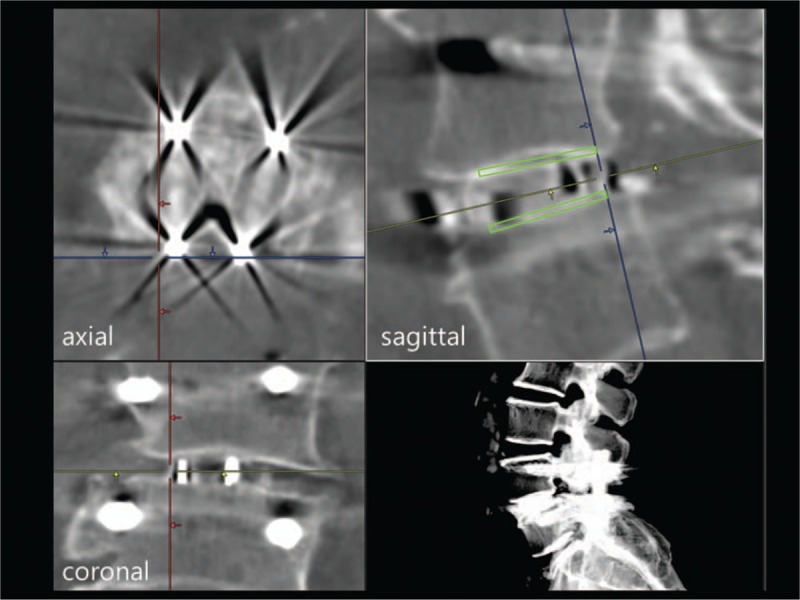
Multiplanar reconstruction image for evaluation of bone ongrowth on a cage surface. A sagittal plane was adjusted to the plane containing the cage frame that formed the lateral wall of each cage. A rectangular ROI (green) was placed on the upper and lower surface of each cage frame with 1-mm height on the sagittal plane (upper right) for calculation of HU values. HU = Hounsfield unit, ROI = regions of interest.
2.4. Qualitative evaluation of bone ongrowth on the surfaces of cage frames by CT color mapping
The CT images were displayed to highlight the regions of bone generation and remodeling with a window width (WW) of 1600 HU and a window level (WL) of 800 HU according to the previous report.[17] Then, the obtained HU values were mapped to a spectral color scale that displayed from dark purple (0 HU) to red (1600 HU), and postoperative color changes on the surfaces of the cage frames were evaluated. If the color tone on the surface of a cage frame changed toward red on the sagittal plane, postoperative bone ongrowth existed on the surface of the cage frame (Fig. 3).
Figure 3.
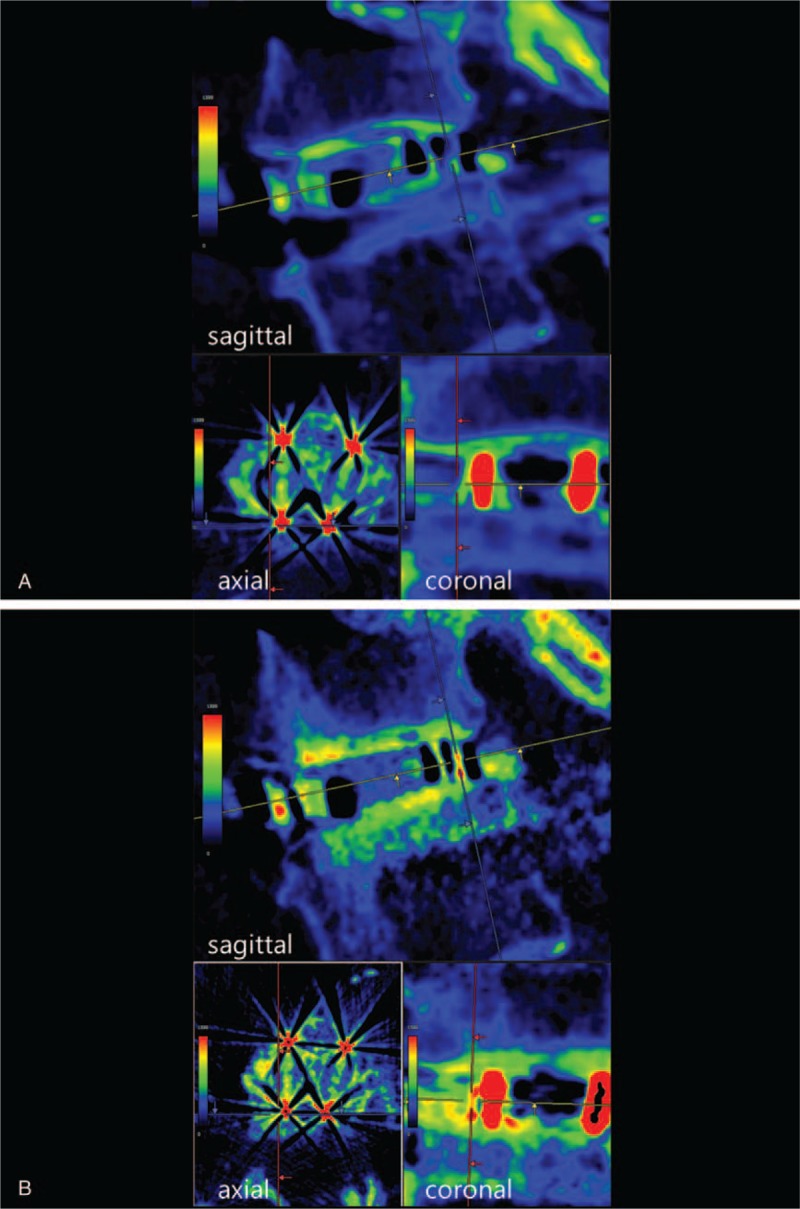
CT color mapping in a 73-year-old woman who underwent L4–L5 posterior lumbar interbody fusion (A: within 1 week postoperatively, B: 6 months postoperatively). In this case, bone ongrowth was observed on both the upper and lower surfaces of the cage frame in the sagittal plane. CT = computed tomography.
For the analysis of intraobserver reliability, the first author evaluated bone ongrowth on 104 surfaces randomly twice with a 2-week interval. For the analysis of interobserver reliability, the first and third authors evaluated bone ongrowth on 104 surfaces randomly.
2.5. Quantitative evaluation of bone ongrowth on the surfaces of cage frames by HU values
The CT images were displayed with a WW of 2000 HU and a WL of 300 HU. The rectangular region of interest (ROI) was placed on the upper and lower surfaces of each cage frame with 1-mm height on the sagittal plane (Fig. 2). The HU values of each ROI were calculated automatically by the imaging software (Synapse Vincent). Four ROIs were evaluated for each cage. In addition, the HU values in the local ROIs where the postoperative bone ongrowth existed on the CT color mapping were also calculated (Fig. 4). The change rate of HU values of each ROI between 1 week and 6 months postoperatively was calculated from the following formula:
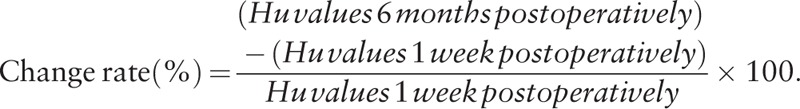 |
Figure 4.
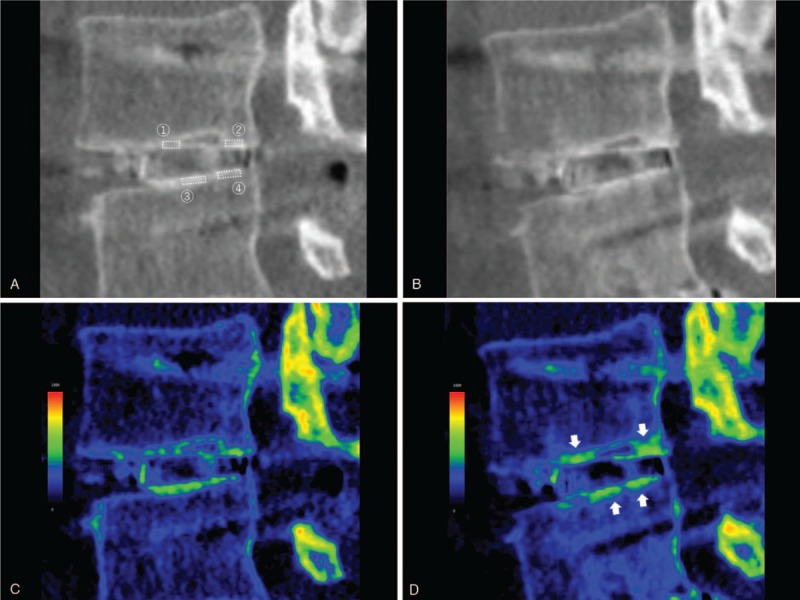
CT in a 69-year-old man who underwent L3–L5 posterior lumbar interbody fusion. Sagittal reconstruction images at the same section at L3–L4 were shown. A, B: gray scale with a WW of 2000 HU and a WL of 300 HU within 1 week postoperatively (A) and 6 months postoperatively (B). C, D: color mapping with a WW of 1600 HU and a WL of 800 HU within 1 week postoperatively (C) and 6 months postoperatively (D). The postoperative local increase in HU values on the upper and lower surfaces of the cage frame, where the presence of bone ongrowth was suggested, could be recognized more easily by CT color mapping than conventional gray scale images (arrows). The ROI 1-4 represented the local ROIs where the postoperative bone ongrowth existed on the CT color mapping. CT = computed tomography, HU = Hounsfield unit, ROI = regions of interest, WL = window level, WW = window width.
2.6. Statistical analysis
Statistical analysis was performed with IBM SPSS Statistics Version 22 (IBM, Armonk, NY). Intra- and interobserver agreement for evaluation of bone ongrowth on the surfaces of the cage frames was assessed with Cohen's kappa coefficient. For univariate analysis, the Mann–Whitney's U test was performed to compare the HU values on the surfaces of the cage frames and change rate of the HU values between the 2 groups. The Wilcoxon signed-ranks test was performed to compare the HU values on the surface of each cage frame between 1 week and 6 months postoperatively. Differences were considered statistically significant at P < .05.
3. Results
Table 1 shows the demographic data from all patients. PLIF was performed on 31 segments, and a total of 62 Ti-coated PEEK cages were inserted in the intervertebral spaces.
Table 1.
Patient demographic and clinical data.

A total of 248 surfaces of the cage frames were evaluated by CT color mapping. Of these surfaces, 134 of 248 (54.0%) were judged to show the presence of bone ongrowth postoperatively and 114 of 248 (46.0%) to show the absence of bone ongrowth postoperatively. Intraobserver reliability for the evaluation of bone ongrowth on the surfaces of the cage frames (n = 104) was kappa = 0.831, and interobserver reliability was kappa = 0.713.
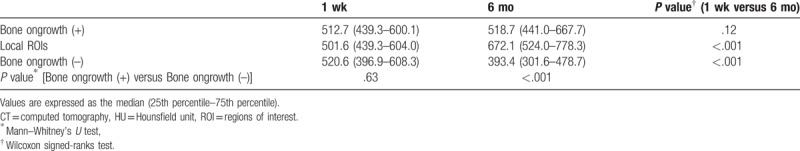
The HU values on the surfaces of the cage frames at 1 week did not differ between frames with bone ongrowth and those without bone ongrowth. In contrast, 6 months postoperatively, the HU values on the surfaces with bone ongrowth were significantly larger than those on the surfaces without bone ongrowth (P < .001) (Table 2). The HU values on the surfaces with bone ongrowth did not show postoperative changes with full length; however, those in the local ROIs where the postoperative bone ongrowth existed on the CT color mapping increased significantly postoperatively (P < .001) (Table 2). In contrast, the HU values on the surfaces without bone ongrowth decreased significantly postoperatively (P < .001) (Table 2). The median change rate of HU values was 2.2% (interquartile range, −11.2%–21.4%) on the surfaces with bone ongrowth; -19.8% (−34.7%–−1.1%) on those without. The change rate of HU values was significantly lower on the surfaces without bone ongrowth than those with bone ongrowth (P < .001). The median change rate of HU values in the local ROIs where the postoperative bone ongrowth existed on the CT color mapping was 22.4% (6.9%–49.0%).
Table 2.
HU values on the surfaces of the cage frames with and without bone ongrowth and in the local ROIs where the postoperative bone ongrowth existed on the CT color mapping.
3.1. Illustrative case (Fig. 4)
A 69-year-old man underwent L3–L5 PLIF. Figure 4 is sagittal reconstruction gray scale and color mapping images of CT scans (Fig. 4a, 4c: within 1 week postoperatively; Fig. 4b, 4d: 6 months postoperatively) at the same section which contains the same cage frame that formed the lateral wall of the cage at L3–L4. The HU values remained unchanged in the rectangular ROI on the upper surface of the cage frame (541.6 HU to 547.1 HU). Furthermore, the HU values decreased in the rectangular ROI on the lower surface of the cage frame (715.2 HU to 654.3 HU). However, the postoperative local increase in HU values at the anterior (577.8HU to 843.6HU, ROI 1) and posterior parts (699.4 HU to 963.5 HU, ROI 2) of the upper surface and at the middle (761.9 HU to 851.2 HU, ROI 3) and posterior parts (777.2 HU to 781.7 HU, ROI 4) of the lower surface of the cage frame, where the presence of bone ongrowth was suggested, could be recognized more easily by CT color mapping than conventional gray scale images.
4. Discussion
This study showed that the qualitative assessment of bone ongrowth on the surfaces of Ti-coated PEEK cages by CT color mapping had adequate inter- and intraobserver reliability. Owing to its easy visualization, CT color mapping could help to detect local increase in HU values on the surfaces of the Ti-coated PEEK cages where the presence of bone ongrowth was suggested, even if the HU values of the rectangular ROIs on the surfaces of the cage frames remained unchanged or decreased postoperatively. To the best of our knowledge, this is the first trial to evaluate bone ongrowth on the surface of Ti-coated PEEK cages by CT color mapping in vivo.
Ti-coated PEEK cages have been developed to give bioactivity and osteoconductivity to the surface of pure PEEK cages while maintaining their radiolucency and elastic modulus.[10] In animal models, several authors have demonstrated osseointegration on the surface of Ti-coated PEEK implants and increased pull-out strength of Ti-coated PEEK implants histologically and biomechanically.[7,18] In contrast, little is known about osseointegration or direct bonding between implant surfaces and vertebral endplates in Ti-coated PEEK cages in humans in vivo and ex vivo, mainly because there are few available methods of evaluation for this concern.
CT studies are considered an available and feasible method for the evaluation of bone ongrowth on the surface of Ti-coated PEEK cages in humans. However, metal artifact is a great concern in the evaluation of the interface between bone and metal implants in CT scans. Cook et al[13] showed that clinical CT tended to overestimate bone ingrowth on metal implants in a canine total hip arthroplasty (THA) model because of the metal artifact of thick implants. In the present study, the layer of Ti coating on a PEEK frame is only 0.065 to 0.15 mm thick. A thinner CT slice is also important for accurate evaluation of the bone-implant interface against metal artifacts.[17,19] In this study, a 0.5-mm or 0.625-mm slice thickness of the CT scan was used. The thinness of the Ti-coated layer and the slice thickness of the CT scans could help to reduce metal artifacts.
The setting of the range of HU values is the other important condition for reduction of metal artifacts and accurate evaluation of bone ongrowth on the surface of Ti-coated PEEK cages. Lengsfeld et al[20] set the threshold HU values between 300 and 1600 HU to delete soft tissue and metal implant artifacts in assessing periprosthetic bone remodeling after THA in vivo. Shinbo et al[17] compared the histology of the grafted bone with HU values on CT scans using a rabbit posterolateral lumbar fusion model. They found that fibrous tissue was shown between 0 and 300 HU, synthesized bone between 300 and 1000 HU, and remodeled grafted bone between 1000 HU and 1600 HU. Based on these reports, the CT images were displayed with a WW of 1600 HU and a WL of 800 HU in the present study.
Owing to its easy visualization of changes in HU values, CT color mapping has been applied clinically to assess bone remodeling, bone or soft tissue structure, and osseointegration to implant surfaces.[14,15,17,21,22] In the present study, the assessment of bone ongrowth on the surface of Ti-coated PEEK cages by CT color mapping had adequate inter- and intraobserver reliability. Moreover, even if the HU values decreased in a rectangular ROI in total, color mapping could easily detect bone ongrowth on the surface of the cage frame where the local increase in HU values occurred (Fig. 4). This could cause postoperative unchanged or decreased HU values in the ROIs, which were judged to show the presence of bone ongrowth in this study.
It was reported that Ti-coated PEEK implants improved shear strength at the bone-implant interface even at 4 weeks after surgery and continued to improve shear strength with time in a sheep model.[7] In the present study, CT evaluation was performed 6 months postoperatively, when osseointegration on the surface of the Ti-coated PEEK cages had already begun. However, only about half of the surfaces had direct bonding between bone and implant in CT color mapping. In an ovine lumbar interbody fusion model, McGilvray et al[23] reported that bone ingrowth into the Ti porous architecture of the Ti-PEEK composite cage was seen in about 40% to 50% of cases at 18 weeks postoperatively, which supported our findings.
There are some limitations to this study. One limitation is that we could not conduct ex vivo direct histological assessment of osseointegration on the surfaces of the Ti-coated PEEK cages. Another limitation is that osteogenic activity might be different among patients due to wide distribution of age and bone mineral density and difference of the amount of local bone graft. The other limitation is that metal artifacts could affect the CT-based qualitative and quantitative analysis of this study, though several measures to reduce metal artifacts were taken. Finally, this was not a comparative study between the Ti-coated PEEK cage and the pure PEEK cage with the same geometry because of the unavailability of the pure PEEK cage. It cannot be determined whether the Ti-coated PEEK cage is superior to the pure PEEK cage in osseoconductivity on the cage surfaces, whereas this present study was primary aimed to establish the evaluation method for bone ongrowth on the surfaces of the Ti-coated PEEK cage.
5. Conclusion
We developed an in vivo method of evaluating bone ongrowth on the surfaces of a Ti-coated PEEK cage by CT color mapping. This assessment had adequate inter- and intraobserver reliability and was well correlated with the quantitative analysis of HU values. As compared with conventional gray scale images, CT color mapping could help to detect local increase in HU values on the surfaces of the Ti-coated PEEK cages where the presence of bone ongrowth was suggested. Qualitative analysis of the surfaces of Ti-coated PEEK cages by CT color mapping based on HU values is an easy and visually comprehensible method for assessment of bone ongrowth in the bone-implant interface.
Acknowledgments
The authors specially thank AOSpine Asia Pacific for financial support of this research (Grant No. AOSJP (R)2017-16).
Author contributions
Conceptualization: Takahiro Makino.
Data curation: Takahiro Makino, Takashi Kaito, Yusuke Sakai, Shota Takenaka.
Formal analysis: Takahiro Makino.
Funding acquisition: Takahiro Makino.
Investigation: Takahiro Makino.
Methodology: Takahiro Makino, Shota Takenaka.
Supervision: Takashi Kaito, Hideki Yoshikawa.
Validation: Yusuke Sakai.
Writing – original draft: Takahiro Makino.
Writing – review & editing: Takahiro Makino, Takashi Kaito, Yusuke Sakai, Shota Takenaka, Hideki Yoshikawa.
Footnotes
Abbreviations: CT = computed tomography, HU = Hounsfield unit, PEEK = polyetheretherketone, PLIF = posterior lumbar interbody fusion, ROI = region of interest, Ti = titanium, WL = window level, WW = window width.
The authors report no conflicts of interest.
References
- [1].Kersten RFMR, Van Gaalen SM, De Gast A, et al. Polyetheretherketone (PEEK) cages in cervical applications: A systematic review. Spine J 2015;15:1446–60. [DOI] [PubMed] [Google Scholar]
- [2].Kurtz SM, Devine JN. PEEK biomaterials in trauma, orthopedic, and spinal implants. Biomaterials 2007;28:4845–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Seaman S, Kerezoudis P, Bydon M, et al. Titanium vs. polyetheretherketone (PEEK) interbody fusion: meta-analysis and review of the literature. J Clin Neurosci 2017;44:23–9. [DOI] [PubMed] [Google Scholar]
- [4].Olivares-Navarrete R, Gittens RA, Schneider JM, et al. Osteoblasts exhibit a more differentiated phenotype and increased bone morphogenetic protein production on titanium alloy substrates than on poly-ether-ether-ketone. Spine J 2012;12:265–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Olivares-Navarrete R, Hyzy SL, Slosar PJ, et al. Implant materials generate different peri-implant inflammatory factors: poly-ether-ether-ketone promotes fibrosis and microtextured titanium promotes osteogenic factors. Spine 2015;40:399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Schimmel JJP, Poeschmann MS, Horsting PP, et al. PEEK cages in lumbar fusion mid-term clinical outcome and radiologic fusion. Clin Spine Surg 2016;29:E252–8. [DOI] [PubMed] [Google Scholar]
- [7].Walsh WR, Bertollo N, Christou C, et al. Plasma-sprayed titanium coating to polyetheretherketone improves the bone-implant interface. Spine J 2015;15:1041–9. [DOI] [PubMed] [Google Scholar]
- [8].Nemoto O, Asazuma T, Yato Y, et al. Comparison of fusion rates following transforaminal lumbar interbody fusion using polyetheretherketone cages or titanium cages with transpedicular instrumentation. Eur Spine J 2014;23:2150–5. [DOI] [PubMed] [Google Scholar]
- [9].Devine DM, Hahn J, Richards RG, et al. Coating of carbon fiber-reinforced polyetheretherketone implants with titanium to improve bone apposition. J Biomed Mater Res B Appl Biomater 2013;101:591–8. [DOI] [PubMed] [Google Scholar]
- [10].Assem Y, Mobbs RJ, Pelletier MH, et al. Radiological and clinical outcomes of novel Ti/PEEK combined spinal fusion cages: a systematic review and preclinical evaluation. Eur Spine J 2017;26:593–605. [DOI] [PubMed] [Google Scholar]
- [11].Mobbs RJ, Phan K, Assem Y, et al. Combination Ti/PEEK ALIF cage for anterior lumbar interbody fusion: early clinical and radiological results. J Clin Neurosci 2016;34:94–9. [DOI] [PubMed] [Google Scholar]
- [12].Rickert M, Fleege C, Tarhan T, et al. Transforaminal lumbar interbody fusion using polyetheretherketone oblique cages with and without a titanium coating. Bone Joint J 2017;99B:1366–72. [DOI] [PubMed] [Google Scholar]
- [13].Cook SD, Patron LP, Salkeld SL, et al. Correlation of computed tomography with histology in the assessment of periprosthetic defect healing. Clin Orthop Relat Res 2009;467:3213–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Meyer J. Visualization of osseointegration of maxilla and mandible dental implants. Int J Comput Assist Radiol Surg 2010;5:69–76. [DOI] [PubMed] [Google Scholar]
- [15].Meyer J, Sheets CG, Earthman JC. Erratum to: visualization of osseointegration of maxilla and mandible dental implants. Int J Comput Assist Radiol Surg 2015;10:1175. [DOI] [PubMed] [Google Scholar]
- [16].Wang D, Künzel A, Golubovic V, et al. Accuracy of peri-implant bone thickness and validity of assessing bone augmentation material using cone beam computed tomography. Clin Oral Investig 2013;17:1601–9. [DOI] [PubMed] [Google Scholar]
- [17].Shinbo J, Mainil-Varlet P, Watanabe A, et al. Evaluation of early tissue reactions after lumbar intertransverse process fusion using CT in a rabbit. Skeletal Radiol 2010;39:369–73. [DOI] [PubMed] [Google Scholar]
- [18].Guyer RD, Abitbol JJ, Ohnmeiss DD, et al. Evaluating osseointegration into a deeply porous titanium scaffold a biomechanical comparison with PEEK and allograft. Spine 2016;41:e1146–50. [DOI] [PubMed] [Google Scholar]
- [19].Kobayashi F, Ito J, Hayashi T, et al. A study of volumetric visualization and quantitative evaluation of bone trabeculae in helical CT. Dentomaxillofac Radiol 2003;32:181–5. [DOI] [PubMed] [Google Scholar]
- [20].Lengsfeld M, Günther D, Pressel T, et al. Validation data for periprosthetic bone remodelling theories. J Biomech 2002;35:1553–64. [DOI] [PubMed] [Google Scholar]
- [21].Peltola EK, Koskinen SK. Dual-energy computed tomography of cruciate ligament injuries in acute knee trauma. Skeletal Radiol 2015;44:1295–301. [DOI] [PubMed] [Google Scholar]
- [22].Turmezei TD, Treece GM, Gee AH, et al. Quantitative 3D analysis of bone in hip osteoarthritis using clinical computed tomography. Eur Radiol 2016;26:2047–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].McGilvray KC, Waldorff EI, Easley J, et al. Evaluation of a polyetheretherketone (PEEK) titanium composite interbody spacer in an ovine lumbar interbody fusion model: biomechanical, microcomputed tomographic, and histologic analyses. Spine J 2017;17:1907–16. [DOI] [PubMed] [Google Scholar]


