Supplemental Digital Content is available in the text
Keywords: diagnosis, older adults, sarcopenia, screening, sensitivity, specificity
Abstract
A new sarcopenia screening tool named Mini Sarcopenia Risk Assessment (MSRA) has recently been developed, which showed a reasonable sensitivity and specificity.
We cross-culturally adapted and validated the Chinese version of the MSRA in a population of community-dwelling older adults.
We conducted a cross-sectional study in a community in Chengdu, China. Older adults aged 60 years or older were included. A Chinese translation of the MSRA was created. The Chinese version of the MSRA included 2 questionnaires named C-MSRA-7 (containing 7 items) and C-MSRA-5 (containing 5 items). For C-MSRA-7 and C-MSRA-5, total scores of ≤ 30 and 45, respectively, indicate that the subject has sarcopenia. Using 4 common diagnostic criteria of sarcopenia (the European Working Group on Sarcopenia in Older People, Asia Working Group for Sarcopenia, International Working Group on Sarcopenia, and Foundation for the National Institutes of Health criteria) as the “gold standard”; the sensitivity and specificity of the C-MSRA-7 and C-MSRA-5 were examined. We applied the receiver operating characteristic curve to compare the overall accuracy of the C-MSRA-7 and C-MSRA-5 for screening sarcopenia.
We recruited 384 participants (mean age: 71.5 ± 5.8 years). Using different criteria as the “gold standard,” both C-MSRA-7 and C-MSRA-5 have acceptable sensitivity (ranging from 78.0% [95% confidence interval [CI]: 66.3–87.7] to 86.9% [95% CI: 75.87–94.2] for C-MSRA-7 and from 80.2% [95% CI: 70.8–87.6] to 90.2% [95% CI: 79.8–96.3] for C-MSRA-5) for screening sarcopenia. However, compared with the C-MSRA-7, the C-MSRA-5 is simpler and has better specificity (ranging from 55.9% to 70.6% for C-MSRA-5; and 38.3% to 41.0% for C-MSRA-7) and overall diagnostic accuracy.
The MSRA scale was successfully adapted to the Chinese language and validated in Chinese community-dwelling older adults. Compared with C-MSRA-7, C-MSRA-5 is the better tool for screening sarcopenia.
1. Introduction
Sarcopenia, a syndrome characterized by loss of muscle mass and function, recently obtained its International Classification of Disease, 10th Revision, Clinical Modification code.[1] This change represents a milestone in understanding and researching sarcopenia. Numerous studies have demonstrated that sarcopenia is highly prevalent and independently associated with many negative outcomes in older adults, such as functional disability, risk of falls, poor quality-of-life, and even death.[2–5] However, because the loss of muscle mass and reduction in strength and/or physical activity in older adults is generally gradual and not noticeable and is even considered a “normal” process of aging, sarcopenia remains under-recognized and under-diagnosed.[6]
Currently, unique consensus criteria and cut-off points for sarcopenia have not been reached. However, there are at least 6 international groups that have developed the diagnostic criteria for sarcopenia, such as the European Working Group on Sarcopenia in Older People (EWGSOP),[7] the Asia Working Group for Sarcopenia (AWGS),[8] the International Working Group on Sarcopenia (IWGS),[9] and the Foundation for the National Institutes of Health (FNIH) Sarcopenia Project.[10] All these groups agree that the diagnosis of sarcopenia should include skeletal muscle mass (measured by computed tomography [CT], magnetic resonance imaging [MRI], or dual-energy X-ray absorptiometry [DXA], and some groups also recommend bio-impedance analysis [BIA]), gait speed (GS), and/or handgrip strength (HS). Nevertheless, these medical devices are not generally accessible in clinical practice, especially in communities and nursing homes. This situation contributes to the under-diagnosis of sarcopenia. Therefore, an easy-to-use, time-saving, and validated tool for screening sarcopenia is required.
As a pioneer in this field, the Strength, Assistance with walking, Rise from a chair, Climb stairs, and Falls questionnaire (SARC-F) developed by Malmstrom and Morley has been validated in different populations.[11–13] However, previous studies demonstrated that the SARC-F showed very low sensitivity, which means a high risk of missing the individuals with sarcopenia. Recently, Rossi et al developed a new screening tool for sarcopenia named the Mini Sarcopenia Risk Assessment (MSRA) questionnaire, which showed an acceptable sensitivity (80.4%) and specificity (60.4%) in their study population.[14] However, these results needed to be validated in different ethnic populations. Sarcopenia is highly prevalent in Chinese community-dwelling older adults.[15,16] It is important to use a brief tool for screening sarcopenia in Chinese older adults. We therefore cross-culturally adapted a Chinese version of the MSRA questionnaire and evaluated the diagnostic accuracy of it in a population of community-dwelling older adults.
2. Methods
2.1. Translation and adaption of the MSRA questionnaire
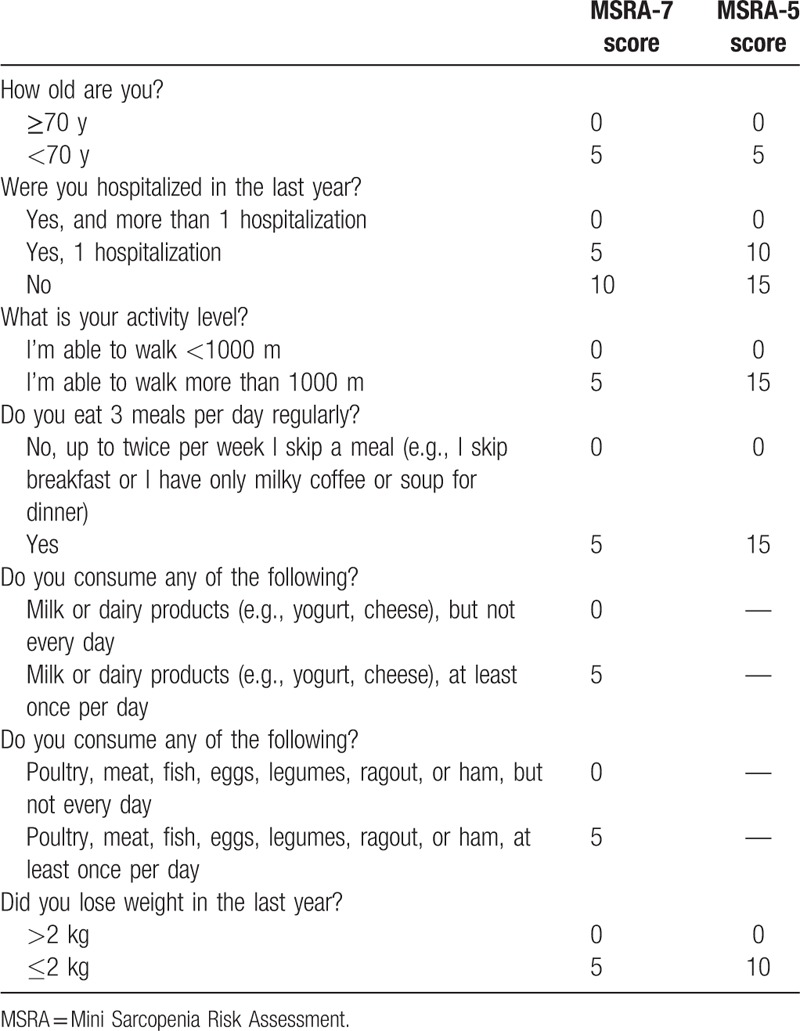
The Chinese version of the MSRA was adapted following a standardized forward–backward translation procedure.[17] The MSRA questionnaire (Table 1) has 2 versions: the full form (including 7 items, named MSRA-7) and the short form (including 5 items, named MSRA-5). Scores of 0, 5, or 10 points are given to the items in the MSRA-7, whereas scores of 0, 5, 10, or 15 are given to the items in the MSRA-5. The total score for the MSRA-7 can be a minimum of 0 points and a maximum of 40 points, and a total score of 30 points or lower indicates sarcopenia. The total score for the MSRA-5 can be a minimum of 0 points and a maximum of 60 points, and a total score of 45 points or lower indicates sarcopenia. The original MSRA-7 and MSRA-5 were independently translated into Chinese by 2 members of our group. Then, our group discussed and revised the translated questionnaires. In item 4 of the MSRA-7, we replaced “milky coffee” with “tea,” because coffee is not a regular food for Chinese people. In item 5 of the MSRA-7, we deleted “cheese,” because cheese is also not a regular food for Chinese people. Next, we asked a native English speaker to perform blind back-translation of the Chinese questionnaires. The back-translated questionnaires were sent to the corresponding author of the original MSRA questionnaire (Dr. Andrea P Rossi). Consequently, Dr. Rossi gave his consent to the Chinese version of the MSRA questionnaires (named the C-MSRA-7 and C-MSRA-5, respectively; Supplementary Table 1). The back-translated version of the MSRA questionnaires is presented in Supplementary Table 2.
Table 1.
The MSRA-7 and MSRA-5 questionnaires.
2.2. Study design and participants
We conducted a cross-sectional study in Shangjin community in Chengdu, China. Written informed consent was obtained from all participants (or their legal proxies). The Research Ethics Committee of Sichuan University approved the study protocol (No. 2017-083). The trained interviewers collected the data from all participants via face-to-face interviews. The anthropometric measurements and other tests were also performed by trained nurses.
During October to November 2017, community-dwelling older adults (aged 60 years or older) were consecutively recruited. Individuals with the following conditions were excluded: medical history of severe mental illness; implanted pacemaker; clinically visible edema; unable to walk; severe renal failure; severe heart failure; and unable to communicate with interviewers.
2.3. Measurement of muscle mass, HS, and GS
The appendicular skeletal muscle mass (ASM) and body fat mass of all participants were measured by using a segmental BIA device (InBody 230, Biospace Co Ltd, Korea). Then, the skeletal muscle mass index (SMI) was calculated using the following equation: SMI (kg/m2) = ASM/height2. The HS of all participants was measured using a handheld dynamometer based on strain gauge sensors (EH101, Xiangshan Inc, Guangdong, China) to the nearest 0.1 kg. Both hands were tested 3 times while the subject was seated with the elbow flexed at a 110° angle, the wrist placed in a neutral position, and the interphalangeal joint of the index finger positioned at a 90° angle. The highest value in either hand was applied for the analyses. The GS was tested by asking the participants to walk a 4-m course at their usual gait. The use of walkers or canes was acceptable, if necessary. All these tests were performed by a trained nurse.
2.4. Assessment of sarcopenia using different criteria
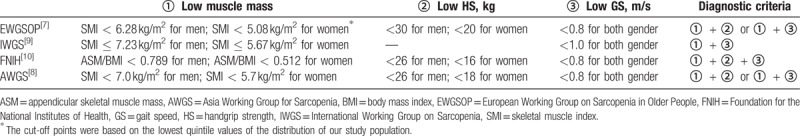
All participants were tested by trained nurses using the C-MSRA-7 and C-MSRA-5 via face-to-face interviews. The nurses who performed the interviews were blinded regarding the results of the aforementioned tests. These tests and the interview for each participant were performed in the same day. For C-MSRA-7, a total score ≤30 indicates that the subject has sarcopenia; for C-MSRA-5, a total score ≤45 indicates the subject has sarcopenia.[14] Additionally, the following criteria for sarcopenia were separately applied as the “gold standard” because these are the most commonly used criteria in sarcopenia research: EWGSOP criteria; AWGS criteria; IWGS criteria; and FNIH criteria. The details of these diagnostic criteria are described in Table 2.
Table 2.
The diagnostic criteria for sarcopenia in this study.
2.5. Covariates
The following covariates were collected from the face-to-face interviews: age, gender, and the medical history of the following chronic diseases: hypertension, diabetes, coronary heart disease, stroke, and chronic obstructive pulmonary disease (COPD). Additionally, body weight and height were measured using a stadiometer and a digital floor scale to the nearest 0.1 cm and 0.1 kg, respectively. The body mass index (BMI) was calculated using the following equation: BMI (kg/m2) = body weight/ height2.
2.6. Statistical analyses
The statistical analyses were performed in MedCalc Statistical Software version 15.2 (MedCalc Software bvba, Ostend, Belgium) and SPSS 20.0 (IBM, SPSS Statistics, Armonk, NY). All statistical tests were 2-sided. A P value of <.05 indicates significance. The results are presented as number (percentage) for categorical variables, mean (standard deviation) for continuous variables with normal distribution, and median (interquartile range) for continuous variables with skewed distribution. For continuous variables with normal distribution, the 1-way analysis of variance was applied to compare the means between participants with or without sarcopenia (based on the EWGSOP criteria). For continuous variables with skewed distribution, a Mann–Whitney test was applied. For categorical variables, chi-squared tests (or Fisher exact test where an expected cell count was <5) were applied. Moreover, classifications using C-MSRA-7 and C-MSRA-5 were compared with the EWGSOP, IWGS, FNIH, and AWGS criteria using chi-squared tests.
Using each of the consensus criteria (Table 2) as the “gold standard,” we examined the sensitivity, specificity, positive likelihood ratio, and negative likelihood ratio of the C-MSRA-7 and C-MSRA-5 for identifying sarcopenia, respectively. Additionally, we applied the receiver operating characteristic (ROC) curve to compare the overall ability of the C-MSRA-7 and C-MSRA-5 questionnaires to discriminate subjects with sarcopenia who were defined by different criteria (Table 2). The area under the ROC curve (AUC) was calculated to measure the concordance of predictive values with actual outcomes. A bigger AUC indicates a better overall diagnostic accuracy.[18] Confidence intervals (CIs) for the AUC and the comparisons between ROC curves were performed using the DeLong method.[19]
3. Results
3.1. The characteristic of the study population
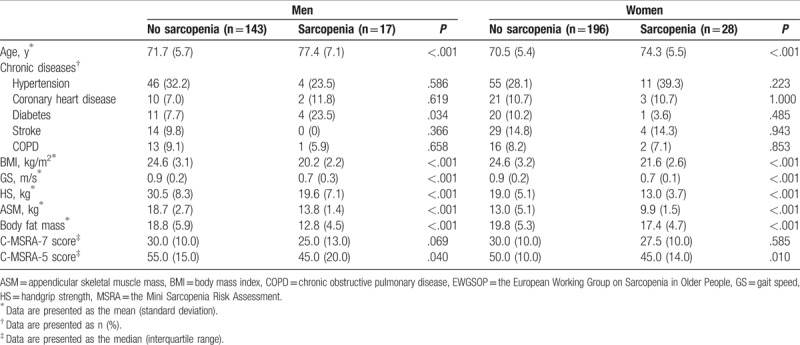
Figure 1 shows the flow diagram of this study. We included 384 participants (160 men and 224 women, mean age: 71.5 ± 5.8 years). Table 3 presents the study population characteristics by gender and the EWGSOP criteria. In both men and women, the BMI, GS, HS, ASM, and body fat mass were significantly lower in the sarcopenia group than in the nonsarcopenia group. In both men and women, the C-MSRA-5 score was significantly higher in the sarcopenia group than in the nonsarcopenia group. However, in both men and women, there was no significant difference between the sarcopenia group and the nonsarcopenia group with respect to the C-MSRA-7 score.
Figure 1.
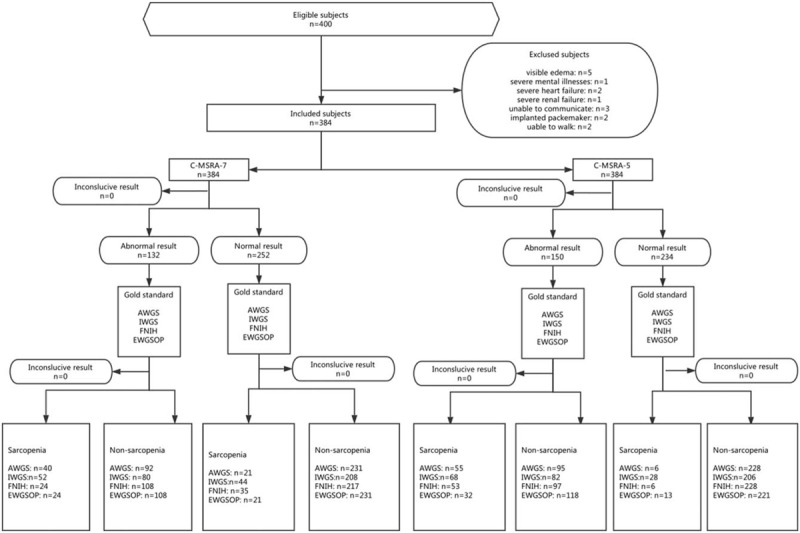
The flow diagram of this study.
Table 3.
Characteristics of the study population according to gender and the EWGSOP criteria of sarcopenia.
3.2. The prevalence of sarcopenia
Using the EWGSOP criteria, the prevalence of sarcopenia was 10.6% and 12.5% in men and women, respectively (P = .573). Using the C-MSRA-7, 28.8% of men and 38.4% of women were classified as having sarcopenia, respectively (P = .050). Using the C-MSRA-5, the corresponding prevalence was 31.9% and 44.2%, respectively (P = .015).
3.3. Comparison of C-MSRA-7 and C-MSRA-5 against different gold standards
The classification of sarcopenia using the C-MSRA-7 or C-MSRA-5 is tabulated according to the 4 consensus criteria (Table 4). Then, each of the 4 criteria was used as the “gold standard”; the diagnostic values of the C-MSRA-7 and C-MSRA-5 are shown in Table 5. The sensitivity and specificity of the C-MSRA-7 were 80.0% and 38.1% against the EWGSOP criteria, 86.9% and 39.6% against the AWGS criteria, 82.3% and 42.0% against the IWGS criteria, and 78.0% and 38.5% against the FNIH criteria, respectively. Similarly, the sensitivity and specificity of the C-MSRA-5 were 82.2% and 65.2% against the EWGSOP criteria, 90.2% and 70.6% against the AWGS criteria, 80.2% and 55.9% against the IWGS criteria, and 89.8% and 68.6% against the FNIH criteria.
Table 4.
C-MSRA and different sarcopenia definitions.
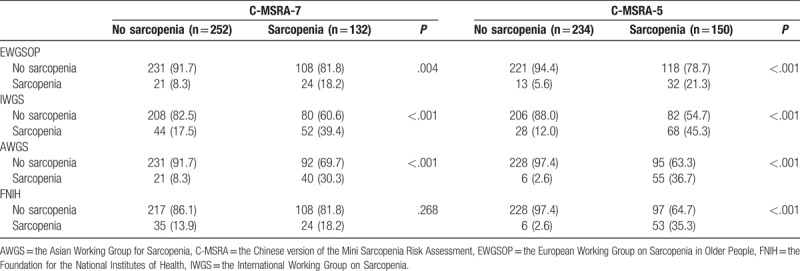
Table 5.
Sensitivity/specificity analyses and receiver operating curve models for C-MSRA-7 and C-MSRA-5 validation against different sarcopenia definitions.
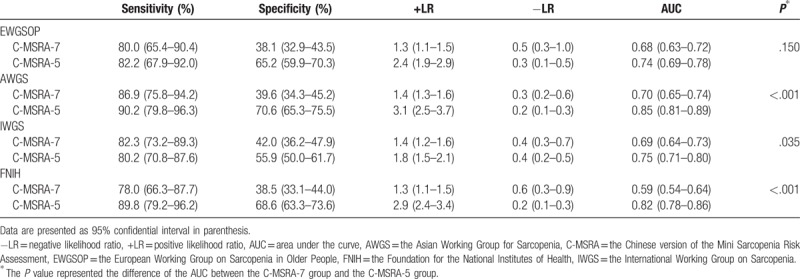
No matter which “gold standard” was used, the AUC of the C-MSRA-5 was better than that of the C-MSRA-7, although the difference was not significant when using the EWGSOP criteria as the “gold standard” (Table 5). Figure 2 shows the ROC curves of the C-MSRA-7 and C-MSRA-5 when using different criteria as the “gold standard.” These findings indicated that the C-MSRA-5 was better than the C-MSRA-7 for screening for sarcopenia.
Figure 2.
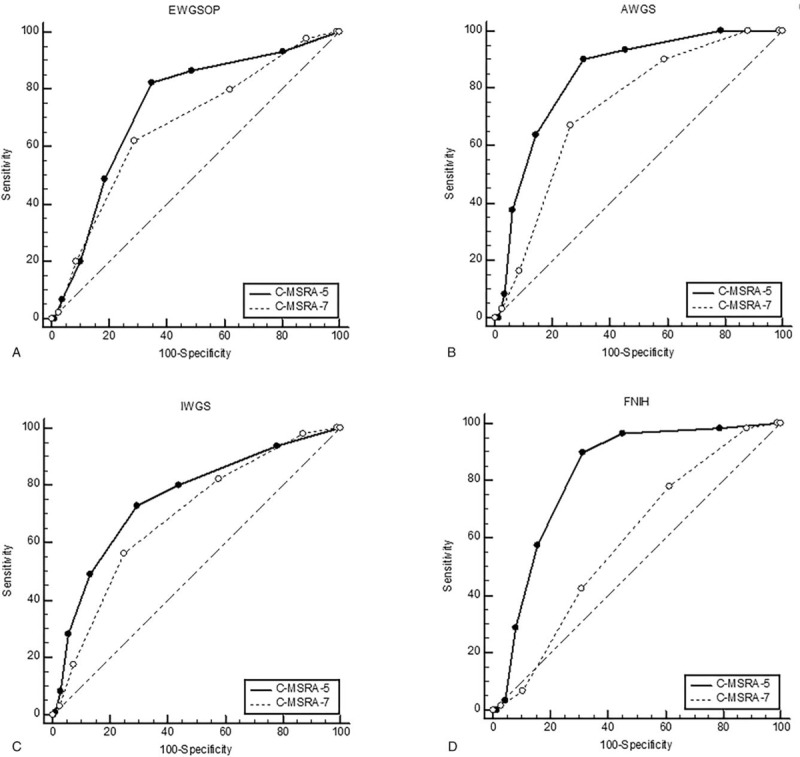
The receiver operating characteristic curves of the C-MSRA-7 and C-MSRA-5 when using different criteria as the “gold standard”: (A) EWGSOP criteria; (B) AWGS criteria; (C) IWGS criteria; (D) FNIH criteria. AWGS = Asia Working Group for Sarcopenia, C-MSRA = the Chinese version of the Mini Sarcopenia Risk Assessment, EWGSOP = European Working Group on Sarcopenia in Older People, FNIH = Foundation for the National Institutes of Health, IWGS = International Working Group on Sarcopenia.
4. Discussion
We cross-culturally adapted and validated the Chinese version of the MSRA, which included 2 questionnaires named C-MSRA-7 (including 7 items) and C-MSRA-5 (including 5 items). Using different consensus criteria as the “gold standard,” both C-MSRA-7 and C-MSRA-5 have an acceptable sensitivity (ranging from 78.0% to 86.9% for C-MSRA-7; and 80.2% to 90.2% for C-MSRA-5) for screening for sarcopenia. However, compared with the C-MSRA-7, the C-MSRA-5 is more concise and has better specificity (range from 55.9% to 70.6% for C-MSRA-5; and 38.3% to 41.0% for C-MSRA-7) and overall diagnostic accuracy. We therefore suggested that compared with C-MSRA-7, the C-MSRA-5 was a better tool for screening sarcopenia.
The original version of the MSRA was developed in community-dwelling older adults in Verona, Italy. Our study validated the MSRA for screening for sarcopenia in Chinese community-dwelling older adults. Further study is needed to determine whether the MSRA is valid in other ethnic populations. Moreover, sarcopenia is highly prevalent in older adults dwelling in long-term care facilities, nursing homes, or hospitals.[2] The validity of the MSRA for screening sarcopenia in these populations also deserves further study.
Our study found that the C-MSRA-5 had similar sensitivity but better specificity and overall diagnostic accuracy than the C-MSRA-7. This finding is in line with the characteristics of the original version of the MSRA.[14] In their study, Rossi et al reported that the sensitivity of MSRA-7 and MSRA-5 was 80.4%, whereas the specificity of MSRA-7 and MSRA-5 was 50.5% and 60.4%, respectively.[14]
The lack of unique diagnostic criteria is one of the most important obstacles in the field of sarcopenia research and implementation of the concept of sarcopenia into clinical practice.[20,21] In this study, we applied 4 common criteria as the “gold standard” to validate the diagnostic value of the C-MSRA-7 and C-MSRA-5. The results of sensitivity and specificity were consistent, regardless of the change in “gold standard.” This finding indicated that the C-MSRA (especially the C-MSRA-5) was a suitable tool for screening sarcopenia.
As the first screening tool for sarcopenia, the SARC-F has been validated in different populations including Chinese older adults.[13,22–24] However, the major weakness of the SARC-F is its low sensitivity, which restricts its use as a screening tool, because low sensitivity indicates high risk of missing subjects who do have sarcopenia. For example, based on a Chinese community-dwelling older population, Woo et al reported that the sensitivity of the SARC-F was only 3.8% to 4.8% in men, and 8.2% to 9.9% in women. Another Spanish study found that the sensitivity of the SARC-F ranged from 28.3% to 35.6% when using different criteria as the “gold standard.”[23] By contrast, both Rossi's study[14] and our study demonstrated that the MSRA had a good sensitivity, either in Italian older adults (both MSRA-7 and MSRA-5: 80.4%) or in Chinese older adults (C-MSRA-7: 78.0–86.9%; C-MSRA-5: 80.2–90.2%). Therefore, the MSRA questionnaire (especially MSRA-5 or C-MSRA-5) appears to be better than the SARC-F for screening sarcopenia in community-dwelling older adults. However, these results come from different study populations. Therefore, a robust conclusion cannot be drawn until head-to-head studies that compare the MSRA and SARC-F in the same study population are conducted.
Our study has some limitations. First, we applied BIA instead of DXA, CT, or MRI to estimate the skeletal muscle mass. The accuracy of BIA for estimating skeletal muscle mass is controversial, and the AWGS and EWGSOP criteria recommend BIA as an alternative option for measuring muscle mass, whereas the IWGS criteria do not. However, this study was conducted in the community; therefore, DXA, CT, or MRI is not suitable for our study population. Second, we only included community-dwelling older adults in this study; whether the C-MSRA questionnaire is suitable for institutionalized or hospitalized elders remains unclear. Third, we only compared the C-MSRA questionnaires with commonly used diagnostic criteria in a cross-sectional study. More importantly, the criterion-related validity of C-MSRA needs to be tested in longitudinal studies in the future. Fourth, we did not test the validity and reliability of the C-MSRA; however, the MSRA has already provided an adequate validity and reliability.[14]
5. Conclusion
The MSRA questionnaire was successfully adapted to the Chinese language and validated in Chinese community-dwelling older adults. The C-MSRA-5 questionnaire is a simple and easy-to-use tool for screening for sarcopenia in community-dwelling older adults. This validation of this tool needs to be confirmed in longitudinal studies with large sample sizes in the future. Moreover, whether the C-MSRA can be applied to screen sarcopenia in institutionalized or hospitalized elders deserves further study in the future.
Author contributions
Conceptualization: Ming Yang, Linna Wu.
Data curation: Lingling Xie, Luoying Zhang, Zengli Han, Linna Wu.
Funding acquisition: Ming Yang.
Investigation: Jie Zhou, Jing Lin, Ying Wang.
Methodology: Ming Yang, Xiaoyi Hu, Jie Zhou.
Project administration: Xiaoyi Hu, Jing Lin, Ying Wang, Yaqi Li, Zengli Han, Daipei Zhang, Yun Zuo.
Resources: Xiaoyi Hu.
Supervision: Xiaoyi Hu, Lingling Xie, Jie Zhou, Ying Li.
Writing – original draft: Ming Yang, Linna Wu.
Writing – review & editing: Ming Yang, Xiaoyi Hu, Lingling Xie, Luoying Zhang, Ying Li, Linna Wu.
Supplementary Material
Footnotes
Abbreviations: ASM = appendicular skeletal muscle mass, AUC = area under the ROC curve, AWGS = Asia Working Group for Sarcopenia, BIA = bio-impedance analysis, BMI = body mass index, CI = confidence interval, CT = computed tomography, DXA = dual-energy X-ray absorptiometry, EWGSOP = European Working Group on Sarcopenia in Older People, FNIH = Foundation for the National Institutes of Health, HS = handgrip strength, GS = gait speed, IWGS = International Working Group on Sarcopenia, MRI = magnetic resonance imaging, MSRA = Mini Sarcopenia Risk Assessment, ROC = receiver operating characteristic, SD = standard deviation, SMI = skeletal muscle mass index.
This study was supported by the Sichuan Medical Association (No. S17054) and the National Natural Science Foundation of China (No. 81501197). The sponsor had no role in the design, methods, data collection, analysis, or preparation of this manuscript.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Anker SD, Morley JE, von Haehling S. Welcome to the ICD-10 code for sarcopenia. J Caxhexia Sarcopenia Muscle 2016;7:512–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Marzetti E, Calvani R, Tosato M, et al. Sarcopenia: an overview. Aging Clin Exp Res 2017;29:11–7. [DOI] [PubMed] [Google Scholar]
- [3].Cruz-Jentoft AJ, Landi F, Topinkova E, et al. Understanding sarcopenia as a geriatric syndrome. Curr Opin Clin Nutr Metab Care 2010;13:1–7. [DOI] [PubMed] [Google Scholar]
- [4].Visser M, Schaap LA. Consequences of sarcopenia. Clin Geriatr Med 2011;27:387–99. [DOI] [PubMed] [Google Scholar]
- [5].Cruz-Jentoft AJ. Sarcopenia: a clinical review. Rev Clin Gerontol 2013;23:267–74. [Google Scholar]
- [6].Woo J. Sarcopenia. Clin Geriatr Med 2017;33:305–14. [DOI] [PubMed] [Google Scholar]
- [7].Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010;39:412–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chen LK, Liu LK, Woo J, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc 2014;15:95–101. [DOI] [PubMed] [Google Scholar]
- [9].Fielding RA, Vellas B, Evans WJ, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc 2011;12:249–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Studenski SA, Peters KW, Alley DE, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci 2014;69:547–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Malmstrom TK, Morley JE. SARC-F: a simple questionnaire to rapidly diagnose sarcopenia. J Am Med Dir Assoc 2013;14:531–2. [DOI] [PubMed] [Google Scholar]
- [12].Wu TY, Liaw CK, Chen FC, et al. Sarcopenia screened with SARC-F questionnaire is associated with quality of life and 4-year mortality. J Am Med Dir Assoc 2016;17:1129–35. [DOI] [PubMed] [Google Scholar]
- [13].Woo J, Leung J, Morley JE. Validating the SARC-F: a suitable community screening tool for sarcopenia? J Am Med Dir Assoc 2014;15:630–4. [DOI] [PubMed] [Google Scholar]
- [14].Rossi AP, Micciolo R, Rubele S, et al. Assessing the risk of sarcopenia in the elderly: the Mini Sarcopenia Risk Assessment (MSRA) questionnaire. J Nutr Health Aging 2017;21:743–9. [DOI] [PubMed] [Google Scholar]
- [15].Han PP, Kang L, Guo Q, et al. Prevalence and factors associated with sarcopenia in suburb-dwelling older Chinese using the Asian Working Group for Sarcopenia Definition. J Gerontol A Biol Sci Med Sci 2016;71:529–35. [DOI] [PubMed] [Google Scholar]
- [16].Gao L, Jiang J, Yang M, et al. Prevalence of sarcopenia and associated factors in Chinese community-dwelling elderly: comparison between rural and urban areas. J Am Med Dir Assoc 2015;16:1003.e1–6. [DOI] [PubMed] [Google Scholar]
- [17].Jones PS, Lee JW, Phillips LR, et al. An adaptation of Brislin's translation model for cross-cultural research. Nurs Res 2001;50:300–4. [DOI] [PubMed] [Google Scholar]
- [18].Linden A. Measuring diagnostic and predictive accuracy in disease management: an introduction to receiver operating characteristic (ROC) analysis. J Eval Clin Pract 2006;12:132–9. [DOI] [PubMed] [Google Scholar]
- [19].DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44:837–45. [PubMed] [Google Scholar]
- [20].Cederholm T, Morley JE. Sarcopenia: the new definitions. Curr Opin Clin Nutr Metab Care 2015;18:1–4. [DOI] [PubMed] [Google Scholar]
- [21].Morley JE. Sarcopenia in the elderly. Fam Pract 2012;29(suppl 1):i44–8. [DOI] [PubMed] [Google Scholar]
- [22].Ida S, Murata K, Nakadachi D, et al. Development of a Japanese version of the SARC-F for diabetic patients: an examination of reliability and validity. Aging Clin Exp Res 2017;29:935–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Parra-Rodriguez L, Szlejf C, Garcia-Gonzalez AI, et al. Cross-cultural adaptation and validation of the Spanish-language version of the SARC-F to assess sarcopenia in Mexican community-dwelling older adults. J Am Med Dir Assoc 2016;17:1142–6. [DOI] [PubMed] [Google Scholar]
- [24].Malmstrom TK, Miller DK, Simonsick EM, et al. SARC-F: a symptom score to predict persons with sarcopenia at risk for poor functional outcomes. J Cachexia Sarcopenia Muscle 2016;7:28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


